![Director of National Parks [logo]](/images/dnp_90px.gif)


![Director of National Parks [logo]](/images/dnp_90px.gif) |
 |
 |
Physical pre-treatment of seed to increase synchronicity, rate and speed of germination
Removal of awns and /or removal or damage of seed coats
Inflorescence and caryopsis parts are known to influence dormancy in many grasses. For example the seed coat can:
In our experiments the removal of seed coats was generally beneficial but may be impractical for some species. Automation of seed coat removal therefore needs to be investigated. These experiments did not investigate the mechanism by which the seed coat retards germination and it is therefore not known if this is a physical or chemical effect, or whether methods other than seed coat removal can overcome the effect. In one experiment the removed seed coats were left in the dish with the seeds to see if there was a leachable chemical inhibitor in the seed coats. There was no effect suggesting the need for the seed coat to be in close contact with the seed whatever the mechanism is.
Removal of these parts may make seed sowing easier but could also have detrimental effects. For example, removal of awns may make it easier to handle the seed and sow uniformly, but they are thought to help pull the seed into the soil. Similarly it is thought that in some species the seed coat can protect against predation and infections.
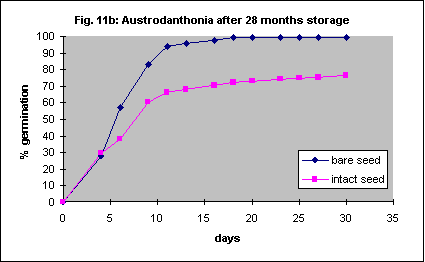
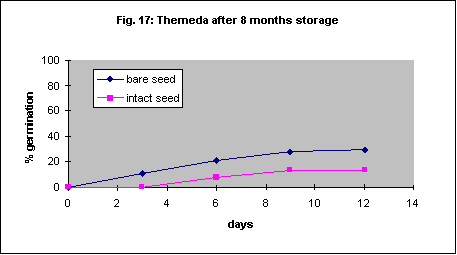
Nine-month-old seed, stored intact at 20° C, was soaked in water for a few hours and the seed coat was removed. Germination of bare seed was much faster but ultimately no more successful when compared with intact seed (Fig. 11a). Older seed germinated more rapidly and more successfully when the seed coat was removed (Fig. 11b). As this species has relatively hard seed it is likely that mechanical removal of the seed coat will be possible.
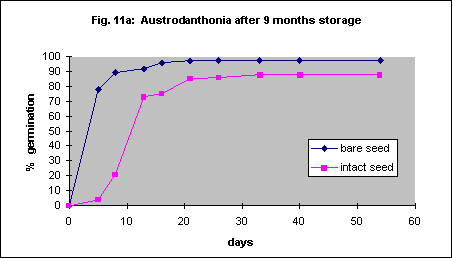
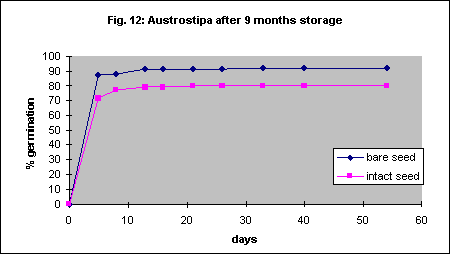
The methods described for Austrodanthonia caespitosa were used, but the seed coats are more difficult to remove. Percent germination significantly increased, though the initial speed of germination was more or less the same. Unlike Austrodanthonia caespitosa the seed of this species is quite soft and it is unlikely that automated seed coat removal will be possible without damaging the embryo. Fortunately the reduction in germination with the seed coat left intact is quite small.
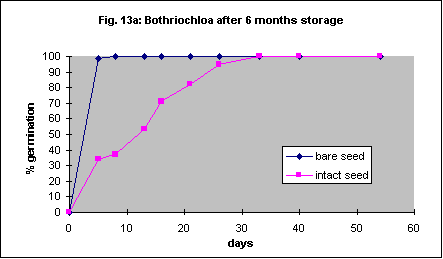
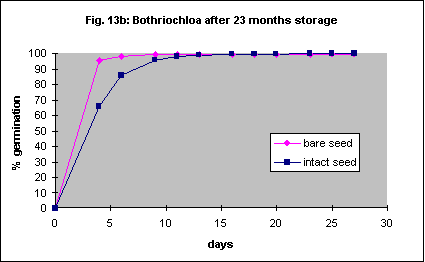
Six-month-old and 23 month old seed was used in this trial but otherwise the methods were the same as for Austrodanthonia caespitosa. With 6 month old seed removal of the seed coat dramatically increased the speed of germination but not the ultimate percentage (Fig. 13a). This effect was not significant with 23 month old seed (Fig.13b). In another experiment with freshly collected seed there was no significant difference between seeds with seed coat on and those with it removed. However the maximum germination percentage was only 51% and the experiment was also badly contaminated with fungi. Like Austrodanthonia caespitosa the seed is quite hard and it should be possible to remove the coat with a machine.
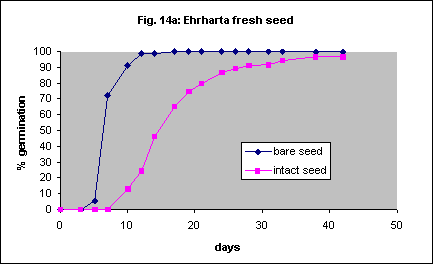
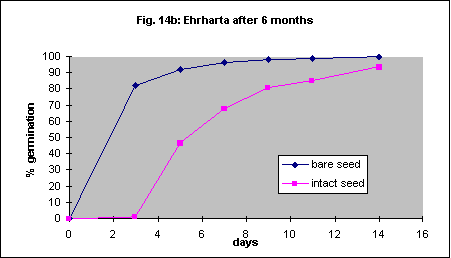
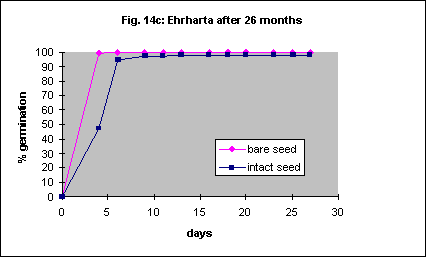
Removal of the seed coat resulted in 100% germination in 10 days with fresh seed. Intact seed only reached a similar percentage after about 40 days (Fig. 14a). Removal of the seed coat gave 80% germination in 3 days in 6 month old seed (Fig.14b). Intact seed took 11 days to reach the same percentage. With 26 month old seed the effect was minimal (Fig. 14c). Automated, mechanical removal of the seed coat should be possible with this species.
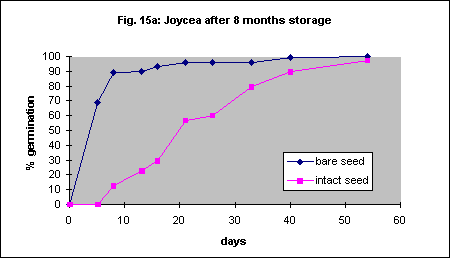
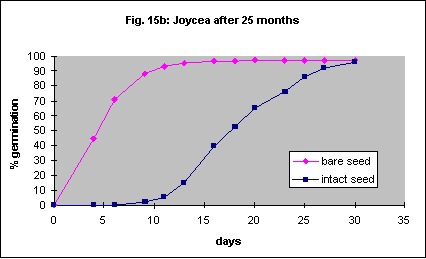
Eight-month-old and 25 month old seed was used using the same methods as above. This is another species that may be amenable to automatic mechanical seed coat removal. Intact seed germinated much more slowly than bare seed regardless of age (Figs. 15a,b).
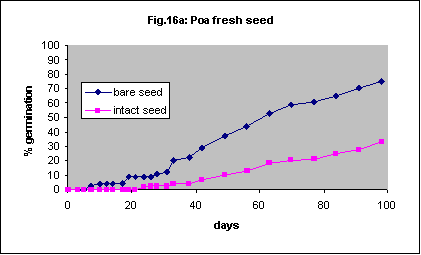
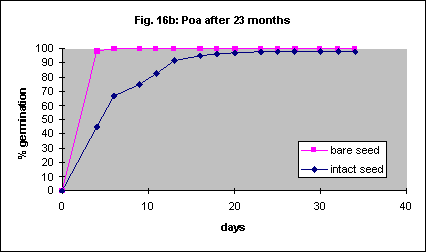
This species has very small seed and it will probably not be feasible to remove the seed coat for field planting despite more rapid germination if it is removed. Final percentage germination of freshly collected seed was increased dramatically by seed coat removal but the speed of germination was very slow (Fig. 16a). With 23 month old seed there was also an acceleration of germination but the final percentage was the same (Fig. 16b).
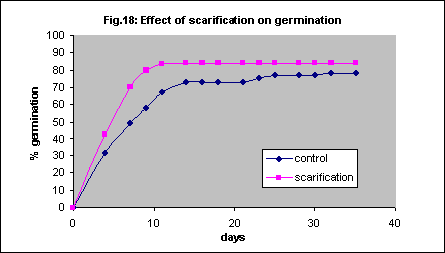
Although the removal of the seed coat is beneficial it is difficult and the seed breaks easily. As an alternative to complete removal scarification, the physical abrasion of the seed coats, is widely used to facilitate germination in many hard seeded species. This increased the initial speed of germination slightly but not the final percentage (Fig. 18).
Effect of light on germination
Light affects dormancy through at least two mechanisms. Red and far red light acts through their effect on the chemical phytochrome, which can either promote or inhibit germination. UV light can have a destructive effect on plant tissues and could break down chemical inhibitors.
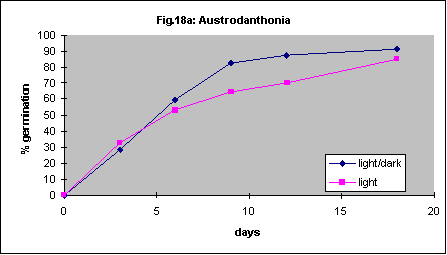
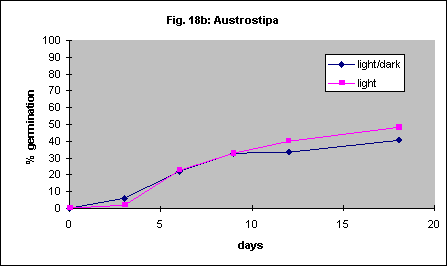
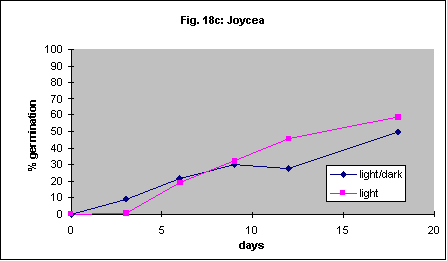
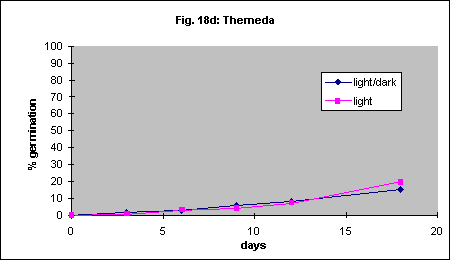
It has been reported that Themeda triandra will not germinate in the dark. One of our collections showed 19% germination after 14 days in the light and 8% in the dark. Another accession germinated poorly regardless of this factor (4% vs. 2%). This probably demonstrates the variability found in different sources of seed. In another series of experiments germination of 4 species in continuous light was compared with germination in a 12-hour light/ 12-hour dark daily cycle. There were no differences in germination speed or percent germination. The results are shown in Figures 18a-d.
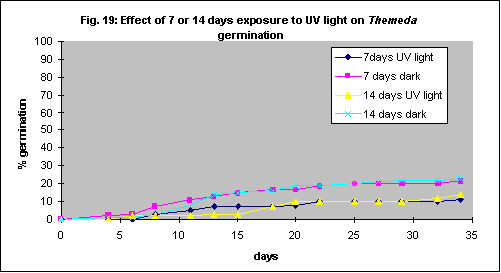
Intact Themeda triandra seed was imbibed in an aqueous solution of 15 mL. L-1 PPM and 4 mL. L-1 7X detergent (wetting agent). It was then placed in petri dishes containing 0.5 mL. L-1 PPM and 4 mL. L-1 7X detergent and incubated 50 cm below a 30 W Sankyo Germicidal UV light for either 7 or 14 days. Control dishes were covered in aluminium foil. UV light reduced germination of Themeda triandra by about 50% (Fig. 19).
Various physical treatments were tested on Themeda triandra. All the seed used in these experiments was imbibed in an aqueous solution of 15 mL. L-1 PPM to minimise pathogens.
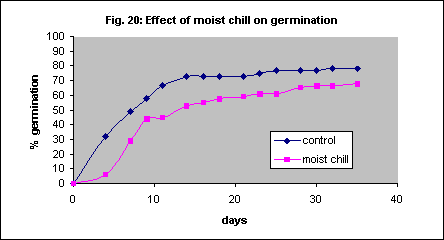
Vernalisation (low temperature and moist conditions) is well known as a promoter of germination in cereal grasses but keeping imbibed Themeda triandra seed for 7 days at 4° C before incubation at 20° C reduced germination (Fig.20).
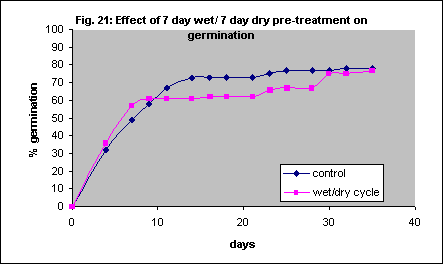
Wet and dry cycles have also been observed to be necessary for germination in some species (eg. Jayasankar et al, 1999) and this environmental condition is likely to be a common occurrence with germinating grass seed. Themeda triandra seed was kept moist for 7 days then allowed to dry for a further 7 days before re-wetting. This did not have any significant beneficial effect, but interestingly was not detrimental either (Fig. 21). Unfortunately we did not have time to test the effects of several wet/dry cycles or different duration of cycles.
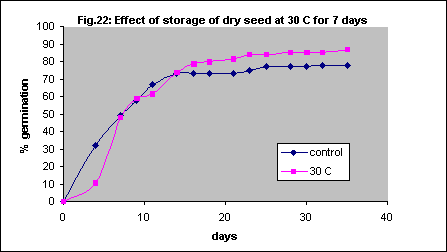
Exposure to high temperature has sometimes been implicated in germination, and germinating seed is likely to be commonly exposed to high soil surface temperatures in the natural environment. However, keeping seed at 30 ° C for seven days prior to imbibition and incubation did not increase germination (Fig. 22). Various other heat treatments were tried without any enhancement of germination. These included microwave heating for 10 or 20 seconds; oven heating at 100° C for 30 seconds or 1 hour; soaking in hot water at 95° C until the water cooled to room temperature; soaking in water at 55–60° C.
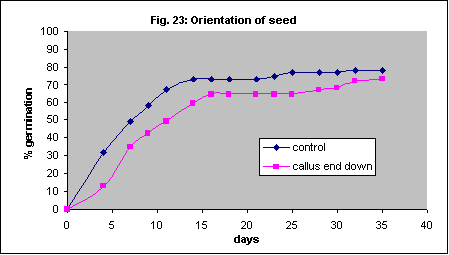
The orientation of the seed in the ground, and humidity conditions surrounding the seed, may influence success. This may relate to the likelihood that most of the water imbibed by a germinating seed enters through the callus. Placing the Themeda seed callus end down in petri dishes did not improve germination, and may have slowed it slightly (Fig. 23). (The other species we used do not have a callus)
[ NEXT PAGE ]