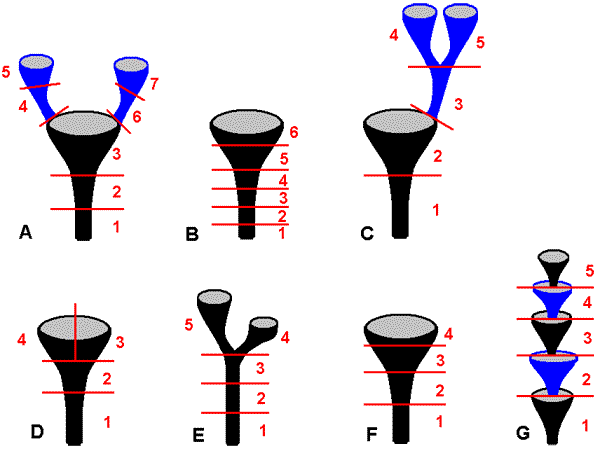
Case StudiesCladonia - quantitative chemical distributionsAlan Archer's paper listed below reports on the quantitative distributions of various lichen compounds in the thalli of specimens of several species of Cladonia. In this genus the thallus develops initially as a squamulose growth form (referred to as the primary thallus) and the primary thalli later produce upright stems, the podetia, as fruticose secondary thalli. The podetia take various forms, depending on species. The specimens tested in the study belonged to what are called scyphiferous species. In such species a podetium expands at the apex into a scyphus, a shallow-cup-like structure. Depending on the species, from such a scyphus a further podetium may develop, which in turn produces a scyphus and so on. The following figures, diagrammatic rather than being to scale, show some of the possibilities. In the study each specimen was cut into sections and the chemical concentrations in each section were analysed. The red lines in the above figures show how the seven specimens were partitioned and the specimens involved were identified as:
The results are presented in the following table. The letters in the leftmost column indicate species, have the same meaning as above, next are the names of the compounds that were found and finally the sectional concentrations. The numbered sections are as shown in the above figures and concentration is given as percentage by weight (w/w on the air-dried thallus).
Archer observed that a conspicuous feature of the data is that the higher concentrations are found in the scyphi (whether terminal or intermediate). He suggested that the localized concentrations in the upper parts of a podetium was caused by repeated upward capillary flow of saturated aqueous solutions of the lichen compounds through the porous medulla, followed by evaporation near or at the podetial apex. Species of Cladonia can absorb water to about 150% of their dry weight, when immersed, and about 50% of their dry weight from overnight dew. Fumarprotocetraric acid has a solubility of 4.5 milligrams per litre at 22 degrees celsius. Hence a 20 milligram portion of moist podetium that contained 10 milligrams of water (namely 100% of dry weight) that was saturated with fumarprotocetraric acid would contain 45 nanograms of the acid, yet the results from the table show that the fumarprotocetraric acid concentration can be up to 5.4% of dry weight, which corresponds to 540 micrograms for 10 milligrams of podetial tissue. This is over 10,000 times the solubility of the acid in water and so the observed concentrations (of at least fumarprotocetraric acid) require a concentrating mechanism, such as that of repeated transport and evaporation as suggested by Archer. Reference
|
![An Australian Government Initiative [logo]](/images/austgovt_brown_90px.gif)