User Documentation for ANHSIR
Australian National Herbarium Specimen Information Register
Written by Julie Matarczyk
February 2004
Contents
Introduction
- Introduction
- The five screens in ANHSIR: LABEL, EVENT, UNIT, ITEM and DET
- The Data Entry Screens: EVENT, UNIT, ITEM and DET
- The Query Screens: LABEL and EVENT
Basic Data Entry – A single Herbarium Specimen
Basic Data Entry – More than one sheet/item
Basic Data Entry – Multiple items, not all herbarium sheets
Data Entry from Field Notebooks
Data Verification
Advanced ANHSIR options
- Label Printing – by Kim Navin
Geocoding – by Lee Halasz
- What is a geocode/Why calculate geocodes?
- Which regions to geocode
- Geocode accuracy and precision
- Geocoding resources
- Geocoding Hints
- Entering the Geocode Related Fields into ANHSIR
- Papuasia - a special region
Troubleshooting…
List of Appendices
- Appendix 1: Function Keys
- Appendix 2: Useful Web Addresses
- Appendix 3: Precision codes - Source codes - Herb material codes - Gardens material codes
- Appendix 4: Geocode ready reckoner
- Appendix 5: Donor Institutes list
- Appendix 6: Abbreviations and Expansions
- Papuasia - a special region
- Botanical Districts of Australia
Frequently asked questions…
Introduction:
The Australian National Herbarium Specimen Information Register (ANHSIR) is a relational database built in Oracle to house information relating to the collections of the Australian National Herbarium (ANH). It is a hierarchical system, allowing information from related collections to be linked together.
The Australian National Herbarium (herbarium code CANB) currently houses collections from two main sources:
- CSIRO’s research based herbarium (herbarium code CANB), and
- the Australian National Botanic Gardens (ANBG) voucher collection (herbarium code CBG).
These two collections were merged to create the current ANH, which retains the herbarium code CANB.
The information currently contained in ANHSIR consists of data from each of these original collections. It combines specimen information from:
- CSIRO’s original specimen register (also known as ANHSIR), and
- the ANBG’s original register (known as IBIS: Integrated Biodiversity Information System).
The five screens in ANHSIR: LABEL, EVENT, UNIT, ITEM and DET
ANHSIR consists of five screens, four of which are utilised during data entry and two of which may be used to query existing information. Data is entered into the EVENT, UNIT, ITEM, and DET screens. When querying an existing record, the information from each of these screens is combined on the LABEL screen. Only the LABEL and EVENT screens may be used to query existing records.
Query Screens |
Data entry Screens |
Label |
Event |
The Data Entry Screens: EVENT, UNIT, ITEM and DET
The four data entry screens are arranged hierarchically such that general information is housed at the top of the hierarchy, more specific information at the bottom. We may think of each of the four data entry screens in the following generalised way:
• EVENT: contains information relating to the locality, the collector and the date. This included geographic information such as Latitude and Longitude.
• UNIT: contains information relating to a particular collection, such as the collector number and habit information.
• ITEM: contains information representing a particular part of the collection, for example, an individual herbarium sheet, a specimen preserved in alcohol, or living material such as cuttings.
• DET: contains all of the information relating to the naming of the collection, including its entire determination history.
ANHSIR is thus designed to allow for more than one DET to be attached to any ITEM, for more than one ITEM to be attached to any UNIT and for more than one UNIT to be attached to any EVENT (see Figure 1).
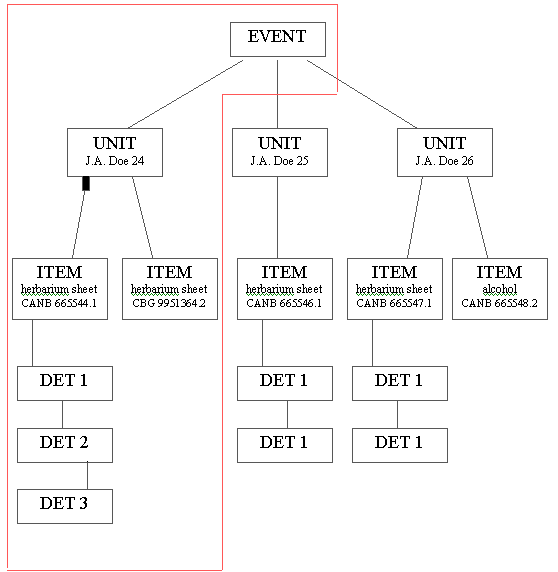
Figure 1: The hierarchical nature of the ANHSIR database. The red line indicates the information that would be returned by a LABEL screen query. An EVENT screen query would return all of this information.
The Query Screens: LABEL and EVENT
Both the LABEL and the EVENT screens may be used to query information already existing in ANHSIR, thus retrieving records for viewing or editing. The LABEL screen shows data from all four entry screens in a format similar to an herbarium sheet label. The LABEL screen query allows users to frame their query in terms of any of the information that is presented on that screen, while the EVENT screen query restricts users to that information which is present on the EVENT screen. This means that querying the LABEL screen allows more flexibility than querying the EVENT screen.
Every record in ANHSIR includes a unique "herb code" and "accession number" combination and this forms the basis of the most common method of querying. Any record that is retrieved via a LABEL screen query will display only the information that is attached to the particular "herb code", "accession number" combination that is queried. An EVENT screen query on the other hand will retrieve the record to which the "herb code" and "accession number" belong, as well as any other information that is attached to that particular record. For example, if there are several UNITs attached to a particular EVENT, a LABEL screen query will only retrieve the information relevant to the desired UNIT, while an EVENT screen query will retrieve the information relevant to each and every one of the UNITs attached to that unique EVENT.
NOTE: to query successfully on the EVENT screen, the LABEL screen must be blank.
Basic Data Entry – A single Herbarium Specimen
To log on to ANHSIR it is necessary to enter a User name, Password, and "ibis".
The LABEL screen is not used during data entry. As described previously it can only be used to query records, or sets of records, for viewing or editing.
The EVENT Screen
Data entry commences with the EVENT screen shown below:
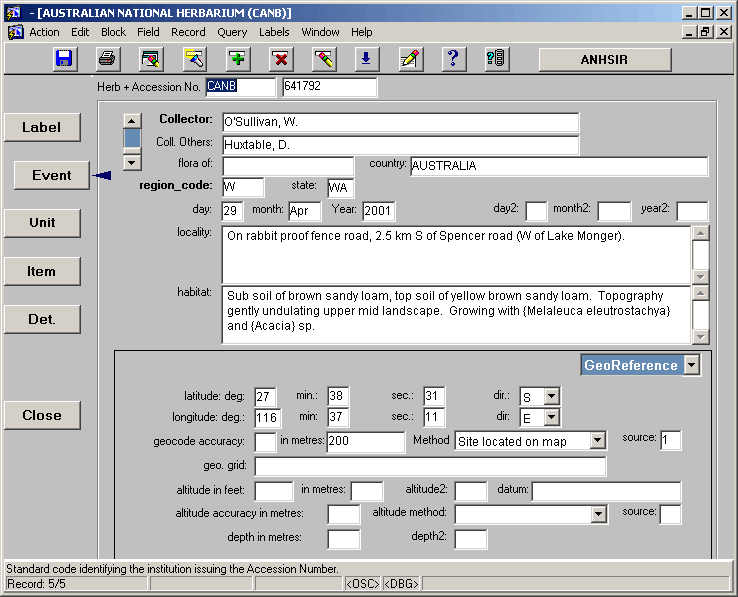
• Herb + Accession No. – The herbarium code and accession number are entered here, e.g. CANB 625278 or CBG 8734976.
NOTE: although the herbarium code and accession number are entered on the EVENT screen, these two pieces of information are not actually stored here. They are stored at the ITEM screen level. Entering this information here allows the database to check whether that particular combination of herb code and accession number already exist in the database, thus preventing the record from unnecessarily being entered twice.
• Collector – The collector field houses the name of the person who collected the specimen, in the following format "Doe, J.A.". There is a look up table associated with the Collector field, which lists collectors and associated collectors. If the collector you require is not listed in the look up table you can add their name, being sure to follow the specified format. Where there are also associate collectors, their names can be added in the following format "Doe, J.A.; Smith, F.E.; Jones, D.R.". It is preferable to list a single "Primary Collector" and list associated collectors in the "Other Collectors" column, however there are occasions where there is more than one Primary Collector and they may be entered accordingly.
NOTE: Where there is a look up table associated with a field, ANHSIR will not accept any entry that is not listed in the look up table. Wildcards (%) may be used when querying look up tables, although they are not necessary at the end of an entry. Use F9 to query any look up table.
• Coll. Others – The names of associate/other collectors are listed in this field. This field is filled automatically according to the selection made from the Collector look up table.
• field number – the field number (often referred to as the "collector number"), is the number allocated by the collector to each particular plant collection they make in the field. This field should display the field number as it is given on the label. For example 873A, 9873, 23b, ANU487, NGF29987. The only time we alter a field number is where the letters preceding the number are the collectors initials. For example, if the collector is D.L.Jones and the field number is DLJ5897, we would enter the field number as 5897. If the collector is J.H.Whinray and the field number is DLJ5899, the letters DLJ must be entered as part of the field number. Entries into this field should appear as they appear on the label, for example, if there is a space between the letters and the numbers on the label, then there should be a space entered between them in the field number field.
Where no field number is provided this field should be filled with the abbreviation "s.n." which represents the latin terms "sine numero" which effectively mean "without number". The field number field should never be left blank.
NOTE: as for the Herb + Accession No., although the field number is entered on the EVENT screen, it is not actually stored here. It is stored at the UNIT screen level. Entering this information here allows the database to check whether that particular combination of collector and field number already exists in the database. If the combination already exists, there may have been an error in numbering, or the record may already be databased. This is an excellent way to check for other sheets of the same collection already existing in the collection/database.
• flora of – This field indicates the area where the specimen was collected, but is only filled in where that information differs from the State, region, and other locality information. As a result this field is rarely used.
• country (default "AUSTRALIA") – The Country of origin of the collection is listed in this field. Note: country should always be filled out in full, e.g. UNITED STATES OF AMERICA not USA; PAPUA NEW GUINEA not PNG.
HINT: the country field is defaulted to capitals, so it is not necessary to hit Caps Lock, or hold down the Shift key when filling in this field.
• region_code – A three letter code for the region where the collection was made. There is a look up table associated with this field. Most region codes commence with the letter which represents the state in which the region occurs, and contain a further two letters to indicate the region, e.g. NST = NSW, Southern Tablelands. Where the region code is not indicated or implied from the label, the letter that indicates the appropriate State is entered into this field. For collections where Australia is the only information given AUS should be entered. For collections made outside of Australia, an F is entered to indicate that the collection is foreign.
(Australian Botanical Regional Code list)NOTE: Since N is used to indicate NSW, the letter D is used at the beginning of codes representing the Northern Territory.
• state – State is the two or three letter abbreviation for each State or Territory. This field is filled in automatically according to the entry made in the region_code field.
• day/month/Year – These three fields are used to indicate the day on which the collection was made. All fields may be entered numerically (the month field will convert to a letter abbreviation upon advancement to the Year field), and where the day and Year field are filled to completion (essential for the Year field) the cursor will advance automatically to the next field. So for a collection made on the 5th of the month, an entry of 05 into the day field will result in the cursor automatically advancing to the month field.
• day2/month2/year2 – These field are available for use where a label indicates a range of time in which the collection was made rather than a specified day, month or year. For example, a label showing 1802-1804 in the date field may be entered as 1802 in the "Year" field and 1804 in the "year2" field.
• locality – This field is used to describe the location of the collection in plain English. For example, "23 km from Braidwood towards Bungendore on the Kings Highway." or "Near bore # 3, Tintarra Homestead.".
NOTE: Information in multi-line fields such as locality should always be entered in sentence case: Capitals at the start of sentences and full stops to terminate.
• habitat – This field is used to describe the aspect, substrate, vegetation type, and associated vegetation of the area where the collection was made.
• latitude. deg/min/sec/dir – These field are used to enter the latitude information associated with the collection site. As with the date fields the cursor will advance automatically through these fields where they are filled.
HINT: the "latitude dir." field is defaulted to S, and will need to be edited for collections made in the Northern hemisphere.
• longitude. deg/min/sec/dir – These field are used to enter the longitude information associated with the collection site. As with the date fields the cursor will advance automatically through these fields where they are filled.
HINT: the "longitude dir." Field is defaulted to E, and will need to be edited for collections from Western longitudes.
NOTE: latitude and longitude information together constitutes what is referred to as a "geocode". See geocoding section for further information.
• geocode accuracy – This field contains a code to indicate geocode accuracy (often referred to as a "precision code". See appendix 3 for a list of available precision codes. This field should only be used where the code entered relates exactly to the desired value in metres. Otherwise this field should be left blank and the value entered manual in the "in metres" field.
• in metres – This field indicates geocode accuracy in metres.
• Method – This field is used to indicate the method used to calculate the geocode. There is a drop down list associated with this field that may be accessed by the arrow keys, or by using the first letter of the entries within it.
• source – This field contains a code used to indicate the source of the geocode. See appendix 3 for a list of available sources.
• geo. grid - This field is used to house information related to grid references, e.g. 3857 38764 AMG. Any information provided should be copied verbatim into this field.
• datum – Where datum is indicated on a label or in a field book it may be entered here.
• altitude in feet – This field is used to enter altitude where it is given in feet.
NOTE: When altitude is entered in feet the value is automatically converted and given in metres as well.
• in metres – This field is used to enter altitude where it is given in metres.
• altitude2 – This field is used to indicate where an altitudinal range is given. The lower limit is indicated in either "altitude in feet" or "in metres" and the upper limit is indicated in this field in metres.
• altitude accuracy in metres – This field is used to indicate the accuracy of the given altitude. This field is only filled in where the accuracy is indicated on the label, e.g. 50 +/- 20.
• altitude method – This field is used to indicate the method used to calculate the altitude. There is a drop list on this field that may be accessed by the arrow keys, or by using the first letter of the entries within it.
• source – This field contains a code used to indicate the source of the altitude. See appendix 3 for a list of available sources.
• depth in metres/depth 2 – These fields are used to indicate depth for aquatic plants.
The UNIT Screen
Once all information relevant to the EVENT screen has been entered we continue down to the UNIT screen, shown below.
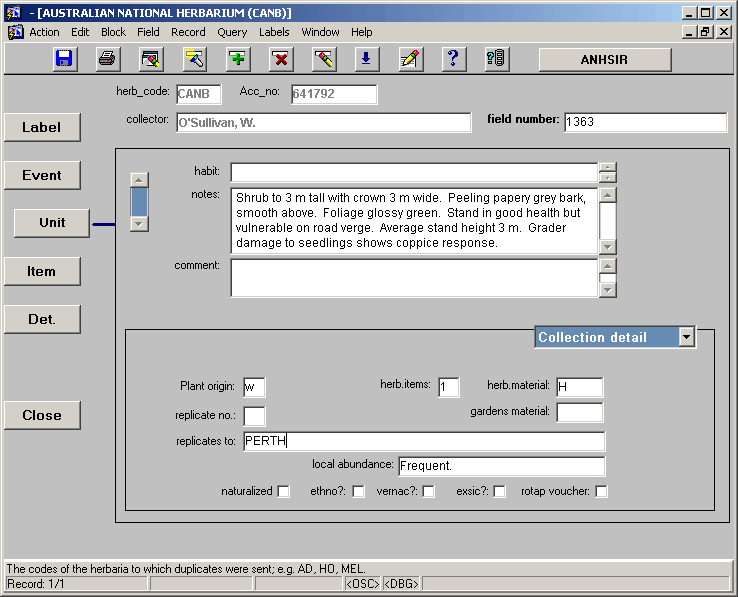
NOTE: Some important pieces of information are displayed on several screens, not just the screen where they are entered. Where this occurs the information is displayed in grey rather than black as an indicator that that particular field is not editable on this screen.
• field number – if the field number was entered at the EVENT screen level it should appear automatically on the UNIT screen. If not, it can be entered at this level.
• habit – the habit field has traditionally been used to enter a brief (usually one word) description of the plants habit e.g. tree; shrub; herb. As the database has developed over the years it has been acknowledged that the information entered into the habit field would usually be qualified or repeated in the "notes" field (see below). As a result, currently no information is entered into the habit field, and this is reflected by the cursor skipping the habit field. The habit field is maintained in the database to accommodate records that are exchanged electronically from other institutes where the habit field is still in use, and to accommodate records where information was entered into the habit field in the past.
• notes – all information which is descriptive of the plant being collected is entered in the notes field. For example "Tree to 40 m tall with yellow flowers and large woody fruit". This field also houses a variety of addition information such as voucher information (including that added at a later date), common names, ethnobotanical information etc.
• comment – the comment field is used to house other information which is relevant to the collection, but perhaps is not specific to the particular collection, or is seen to be additional to the notes. For example, if a collection was made as part of specific expedition for which there is information given about funding or support; or if the collection was made as part of specific study, e.g. "Collected as part of the White-tailed Black Cockatoo Survey…", this information would be entered in the comment field.
• plant origin – this field is used to indicate the origin of the material from which the collection was made. This is indicated with a single letter code; w = material is of wild origin, c = material is of cultivated origin with no information as to the source, o = material is of cultivated origin with information as to the wild origin, u = origin of material is unknown or ambiguous.
• herb items – a number is placed in this field to reflect the number of herbarium items which exist for a particular collection. The most common scenario is a single herbarium sheet, therefore the most common entry in this field is "1". However, any number of items may exist, for example, a collection may consist of 2 herbarium sheets and a specimen stored in alcohol, which would require a number of "3" in the herb items field.
• herb material – letters representing the types of herbarium material which exist are placed in this field (see appendix 3 for codes). Letters are entered in upper case, without spaces. For example, a collection that consisted of a herbarium sheet, a specimen in alcohol and a floral card would be coded as "HAK".
• gardens material – letter representing the types of living material taken as part of the collection are entered here (see appendix 3 for codes). Letters are entered in upper case, without spaces. For example, a collection that consisted of cuttings and seed would be coded as "CS". It is important to note that the gardens material field should only be filled in where living material has been passed on to the Australian National Botanic Gardens. Therefore this field will rarely be used when databasing exchange specimens, since any living material that was collected was more than likely kept locally.
• replicate no. – this field allows a complier to indicate how many replicate labels will be required, to send out with duplicate specimens. Generally this field will only be used when entering data from field books.
• replicates to – the destination of duplicate specimens of this collection which were distributed to other herbaria or institutions is recorded here. It is preferable to use herbarium codes (as per Index Herbariorum) where possible. Codes are entered in upper case, separated by a comma and a space. For example if 4 duplicates were sent to herbaria in Melbourne, Sydney, Brisbane and Kew this field would be filled as "MEL, NSW, BRI, K".
NOTE: The information in the replicates to fields should reflect that which is provided on the label. It is not necessary to make assumptions about replicates e.g. we have a specimen so there must have been one sent to CANB. The only replicate information that is not entered into these fields is where it is indicated that there is a specimen in "CANB only". Where this is the case the fields should be left blank.
• local abundance – any information which relates to the abundance of the specimen which was collected is entered in this field. This can range from a single word description such as "common" or "occasional", to more detailed descriptions such as "rare, only 2 plants found in this area". Where possible these descriptions should be separated from habitat information, provided that this does not result in a loss of information due to a lack of context. Occasionally it is necessary to repeat this information in both the notes field and the local abundance field for clarity. For examples, if a label stated "common in this area but none seen in similar habitat a few kilometres up the road", it would be best to put this whole statement into the notes field and put "common in this area" into the local abundance field.
NOTE: the local abundance field has character restriction, which may necessitate the inclusion on information into the notes field
• naturalized – this field is a tick box which should be checked if the label states that the species collected is naturalized in the area.
• ethno? – this field is a tick box which should be checked if there is some ethnobotanical information provided for the plant collected. For example if a label states "this species used for medicinal purposed by local tribes" the information should be entered in the notes field and the ethno? box should be ticked.
• vernac? – where common, vernacular or local names are given for the species collected the information should be entered in the notes field and the vernac? box ticked.
• exccic? – commonly used in cryptogams this box should be checked when the collection is part of an exccicatae set.
• rotap voucher – ROTAP stands for Rare Or Threatened Australian Plants. Although the ROTAP list is no longer in use, this check box is present in ANHSIR to accommodate historic information. It should not be necessary to check this box while performing contemporary data entry.
The ITEM Screen
Once all information relevant to the UNIT screen has been entered we continue down to the ITEM screen, shown below.
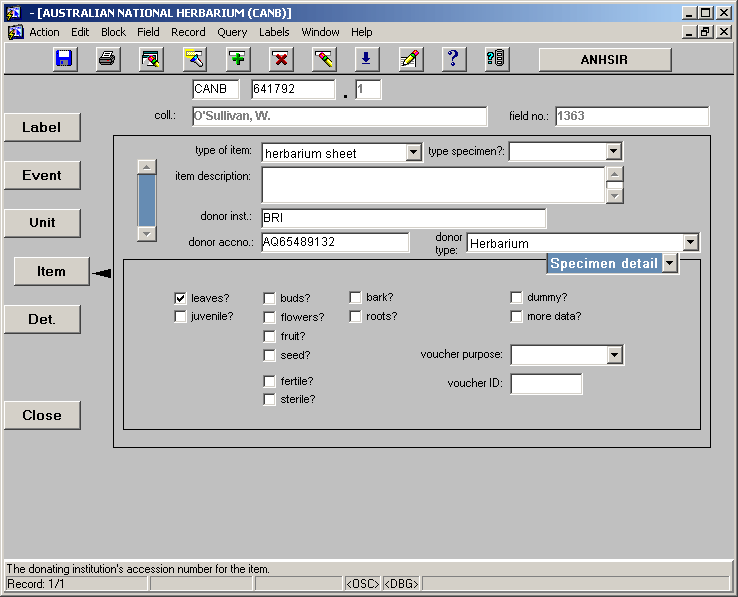
• Herb + Accession No. – if the herbarium code and accession number were entered at the EVENT screen level they should appear automatically on the ITEM screen. If not, they can be entered.
• type of item – there is a drop box associated with the type of item field which allows a choice of the type of item being entered. The most common selection from this box is "herbarium sheet".
• type specimen – if the item being databased is a TYPE specimen it is indicated in the type specimen field. Options such as HOLOTYPE, ISOTYPE and LECTOTYPE are available from the drop box associated with this field.
• item description – this field should be used to indicate anything particular or peculiar about the item being databased which will not be apparent from the fully databased record. For example, if a herbarium sheet has photographs attached to it a comment such as "photographs attached" could be added.
• donor inst – this field is used to indicate the donor institute where the specimen being databased was not collected by the home institute, for example, material which has been donated via exchange with another institute. Where possible, this should be indicated by the formal herbarium code of the donor institute, such as MEL, BRI, K, or L. Where the donor does not have a formal herbarium code there are a number of options available to indicate who the donor was. Appendix 4 contains a list of alternative donor institute codes that can be useful. Where the donor is an individual this should be indicated by the persons name in the follow format "J.A.Doe". If the donor does not fall into any of these categories there is a Donor Institutions list that may be accessed for alternatives, it is included as Appendix 5.
• donor accno – this field is used to house the accession number used by the donor institute for this particular collection. The accession number should be entered as it appears on the label. For example, many BRI labels list their accession number and generally they are preceded by an "AQ", therefore a BRI accession number should be entered as "AQ28576".
• donor type – the type of institute which donated the specimen should be indicated in this field. There is a drop box available listing many institute types, including herbarium, botanical garden, and university.
NOTE: Any institution that has an Index Herbariorum code should be listed as an Herbarium, even if it is a University, Botanic Garden etc.
• leaves? – flag this box if the specimen has leaves.
• juvenile? - flag this box if the specimen consists of juvenile material.
Note: a specimen may be flagged as being juvenile either because it is apparent from the material on the herbarium sheet or because it is indicated on the label.
• buds? - flag this box if the specimen has buds.
• flowers? - flag this box if the specimen has flowers.
• fruits? - flag this box if the specimen has fruit.
• seed? - flag this box if the specimen has seed.
• fertile? - flag this box if the specimen is fertile.
Note: this field is flagged automatically if any of the buds, flowers, fruits, or seed fields are flagged, but can be flagged independently if the nature of the fertile material is not apparent, such as with grasses.
• sterile? - flag this box if the label on the specimen indicates that the material is sterile.
• bark? - flag this box if the specimen has bark.
Note: this field should only be flagged if there is a substantial piece of bark attached to the sheet, where the bark is present on a stem the field is not flagged.
• roots? – flag this box if the specimen has roots.
• dummy? – flag this box if the sheet is a dummy sheet, for example, a marker for a Type specimen or a floral card in the collection.
• more data – flag this box if there is some form of data presented on the sheet which can not be represented in the database record. For example, if there is a map on the label showing where the specimen was collected, or sketches or drawings made on the herbarium sheet. Where this occurs the type of data presented may be described in the ‘item description’ field.
• voucher purpose – if the specimen is a voucher for DNA, an illustration, a phytochemical study etc, it should be indicated in this field. The type of voucher can be selected from a drop down list.
• voucher ID – where a voucher identification number is indicate on the label or slip it should be entered in this field.
The DET Screen
Once all information relevant to the ITEM screen has been entered we continue down to the DET screen, shown below.
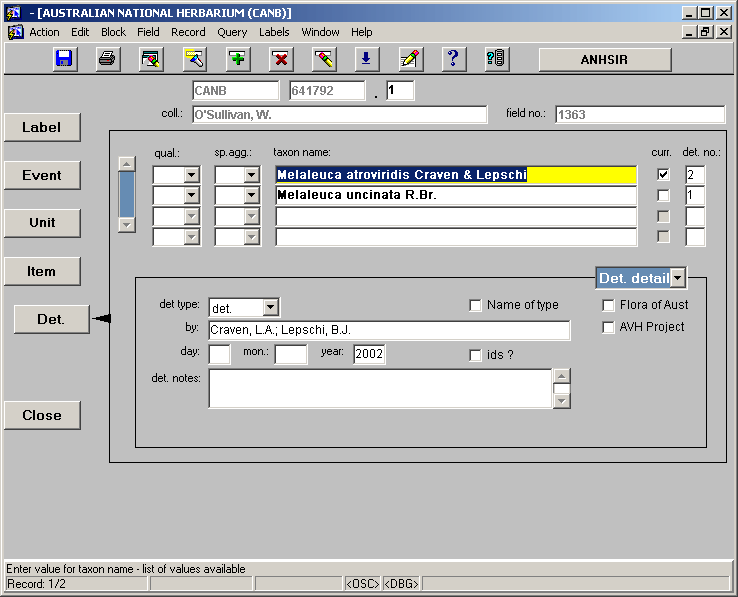
NOTE: The DET screen is quite different from each of the other screens we have looked at previously in that it is able to display multiple records on a single screen. This is so that we can view several or all of the taxon names that make up the determination history at once. Each individual taxon name entered here is associated with its own det. detail and this detail is displayed when the relevant taxon name is highlighted.
• taxon name – each of the taxon names which have been applied to the specimen being databased are entered here. They should be entered one at a time, in chronological order from earliest to most recent. There is a large look up table associated with this field, called the Plant Name Table.
NOTE: The Plant Name Table is important as it also forms the basis of another database known as APNI, the Australian Plant Name Index.
• det type – there is a drop box associated with the det type field which allows a choice of the type of det being entered. The most common selections from this box are "det." and "conf.".
• by – the name person who applied the determination should be entered here, in the same format as for collectors e.g. Doe, J.A.; or Doe, J.A.; Smith, F.E.; Jones, D.R.
• day/mon/year – the date that the name was applied should be entered here.
• det notes – any notes associated with the determination should be entered here. For example, comments such as "this specimen has rather long leaves for this species, perhaps a hybrid".
• Flora of Aust. – flag this box where the det slip indicates that the specimen was determined as part of a study contributing to the Flora of Australia project.
• AVH project – flag this box where the det slip indicates that the specimen was determined as part of the Australias Virtual Herbarium project.
• Name of type – flag this box where the specimen being databased is identified as being a type of the taxon name being entered.
• ids ? – this box is used when databasing redeterminations and is not relevant to basic dataentry.
• qual. – there is a drop box associated with the qual. field which allows any qualifiers of the taxon name, such as "aff.", "?", "c.f.", to be indicated.
• sp. agg. – abbreviation for the latin term sens. lat. and sens. stricto (in the broad sense, and in the narrow/strict sense respectively) as contained in the drop box associated with this field.
• curr. – this box is flagged automatically for the most recently entered, and therefore current, determination. This field may be altered manually where required.
• det. no. – this field is filled automatically as determinations are entered to indicate the order in which they were entered.
When the DET screen is complete pressing F10 will save the entire record. To enter a new record, return to the EVENT screen and press F6, and a new set of entry screen will be presented.
Basic Data Entry – More than one sheet/item
It is fairly common for a herbarium collection to consist of more than one sheet. If a collection consists of two herbarium sheets it should be entered in to ANHSIR in the following way: the first sheet of the collection should be entered as per the instructions for a single sheet with the exception that the ‘herb.items’ field on the UNIT screen should be entered with a 2 rather than a 1.
Once all four screens have been completed, return to the ITEM screen and insert a new item by pressing F6. This creates a second ITEM screen and subsidiary DET screen within the record. This second ITEM may then be completed with regard to the second sheet. Where there is information common to both sheets, for example the donor institute and donor accession number, this information may be copied from the previous ITEM by pressing F3 at the appropriate fields. Where there is little difference between the two ITEMs the entire record may be copied by pressing F4. Once the ITEM information has been entered the DET screen information may be entered.
NOTE: DET screen information should only be entered where it differs from that on the first sheet. If the determination histories are identical the information need not be entered a second time.
These basic adaptations apply to any collection with multiple sheets, whether it be 2 sheets or 12. ANHSIR will allow the addition of as many new ITEMs as are necessary.
Basic Data Entry – Multiple ITEMs, not all herbarium sheets
Where a collection consists of multiple items which are not all herbarium sheets, some small adaptations, similar to those described for multiple sheets, may be used to reflect this.
Usually there will be at least one herbarium sheet associated with a collection and this should be entered as per the instructions for basic data entry of a single sheet with the exception that ‘herb.items’ on the UNIT screen should reflect the total number of herbarium items in the collection, and ‘herb.material’, also on the UNIT screen, should reflect each type of item in the collection. For example:
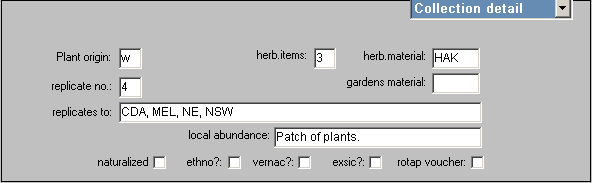
Where
H = an herbarium specimen
A = a specimen stored in alcohol (spirit collection)
K = a floral card
Once the herbarium sheet has been entered, return to the ITEM screen and create a new ITEM by pressing F6. The new ITEM screen should then be completed with regard to the next item.
NOTE: it is important to complete the ‘type of item’ field correctly by selecting alcohol specimen or floral card or herbarium sheet from the drop box as appropriate.
As for multiple sheets, the DET screen should only be completed where the determination history differs from the first item. This process should be repeated for each item of the collection.
Data Entry from Field Notebooks
There are a few things to note when data is being entered from a field notebook rather than a herbarium specimen. Firstly, where latin or scientific plant names are used in fields such as habitat and notes, they must be placed inside curled brackets {} so that when labels are printed the names appear in italics.
Secondly, where a name is given for the plant collected, but is not qualified with a determinative or a determination date, "field" should be selected from the ‘det type’ drop box to indicate that it is a field determination. The determinative need not be added as for a field determination it is assumed that the collector/s is/are the determinative/s.
Thirdly, it should be noted that it is possible when entering from a field notebook, to come across a collection which consists of only living material, such as cuttings or a transplant. This should be reflected by leaving ‘herb.items’ and ‘herb.material’ blank and filling in the appropriate codes in the ‘gardens material’ field on the UNIT screen. Where this occurs there are appropriate options, such as plant, cutting, and seed in the ‘type of item’ field on the ITEM screen.
NOTE: where a collections consist of living material only, draft labels will be produced, but final labels will not.
Linking Records
When entering data from field notebooks it is often highly appropriate to link records together. For example, if the collector has collected several plants from a single location it is possible to link these collections together. This is achieved by entering the first collection fully, but when entering the second collection, rather than returning to the EVENT screen and pressing F6, it is possible to return to the UNIT screen, press F6 and enter the second collection from that point. Pressing F6 at the UNIT screen level creates a new UNIT, ITEM and DET attached to the previously entered EVENT.
NOTE: this should only be done where the EVENT screen details are identical for both records.
This process can then be repeated for all of the collections taken from that location. The result will be several sets of UNIT, ITEM and DET attached to a single EVENT.
NOTE: this method of linking records can also be used when entering data from herbarium sheets, however, it is highly unusual to encounter consecutive collections by a single collector when entered data from herbarium specimens.
Data Verification
Data entry also includes editing and updating existing records. There are several things to be aware of when verifying specimen data. The best general rule of thumb is to ask the question "which parts of this record differ from the way that I would have databased them?" Most of the time these differences need to be rectified. Generally the need for verification arises from errors which were generated when data from the previous ANHSIR and IBIS databases was loaded into our current ANHSIR database. The following lists highlight common changes which should be made to existing records.
NOTE: records without geocodes must have a geocode added where possible.
Common changes to CANB records:
- insert spaces where they have been omitted.
- insert the region code for foreign specimens.
- transfer regions from the written locality information into the region code field.
- move habitat information from the notes field to the habitat field.
- add lat. long. data to the georeference area and delete from the notes field.
NOTE: the previous ANHSIR database did not have fields to accommodate seconds. As a result, geocodes which were accurate to seconds had to be repeated in full in the notes field.
- insert geocode accuracy, method and source.
- insert altitude method and source.
- check the field number.
- move information in the habit field into the notes as appropriate.
- check all information in the "Collection Detail" box. Usually plant origin, herb items, herb material, replicates no, and replicates to will need to be edited.
- if the donor accession number is exactly the same as the CANB number, and does not appear on the label, remove it.
- tick the appropriate boxes under specimen detail.
- re-build the determination history if necessary, by removing the current determination and entering each determination chronologically (the previous ANHSIR only retained the most recent determination).
- check that dates on determinations appear in full.
Common changes to CBG records:
- add geocodes to records which don’t have them.
- check the field number.
- cut and paste abundance information from the notes field to the local abundance field.
- complete the plant origin field if not done so already.
- tick the appropriate boxes under specimen detail.
LinkedRecords
When the data from the old ANHSIR system was loaded into the new ANHSIR database, part of the process was for records containing the same information to be linked together. In most cases this linking was valid and useful, however, there were occasions where this resulted in inappropriate linking of records, either at the EVENT or UNIT level. A common example is that for s.n. numbers. When a collector does not provide a field number we complete the database with the abbreviation "s.n." to indicate that the collection is without a number. There are many cases in the database when several different collections, all with s.n. numbers, have been linked together at the UNIT level in the database. The inappropriate linking becomes apparent when we see that each of the individual ITEMS which are linked to the UNIT in question have different DETs, thus indicating that they are different species and different collections. In such cases it is necessary to separate these records so that each individual collection is represented by a unique UNIT.
Where the number of linked records is small, they may be separated manually by deleting the superfluous details from one record and creating a new one. Where there are many records linked together it is preferable to have them separated through an automated process.
Records may require separation at one of two different levels. Multiple ITEMs may each require their own UNITs, or multiple UNITs may each require their own EVENTs. It is important to specify the necessary level of separation if utilising the automated method of separation.
Recognising Linked Records…
There are several occurrences which may indicate to you that you are dealing with linked records. If you have retrieved your record via a LABEL screen query it will not be apparent from the resulting screens that the record is linked. However, if you happen to be editing a series of records which are linked together it will usually become apparent that the edit has "already been made", or you may recognise an EVENT or UNIT as being "familiar". Although it is difficult to describe, such "warning bells" are easily recognised by technicians who have undertaken large amounts of dataentry.
An easier way of identifying linked records is to retrieve your record via an EVENT screen query rather then a LABEL screen query. Under these circumstances, all of the information attached to the record you are interested in will be displayed, including any inappropriately linked records.
NOTE: it is important to note that because an EVENT screen query retrieves all of the information attached to the desired record, it is possible to find yourself editing the wrong record. For queries of this type it may be necessary to scroll through a number of UNITs or ITEMs to find the desired record.
Advanced ANHSIR options
Label Printing:
One of the primary reasons for entering collection data into ANHSIR is to record information for new accessions to the collection and print standard labels for new specimens before they are stored in the collection. The data may have been entered from field books or have come in hard copy or digital form from other herbaria or individual collectors. ANHSIR facilitates the printing of a number of different label types from the ‘Labels’ option on the main menu at the top of the main form.
Types of labels:
The labels menu contains the following options:
Draft labels: Herbarium sheet labels (without the herb code and accession number barcode) used for checking data entry, geocodes and determinations before the final labels are printed. These labels can be printed on non archive paper or paper already printed on one side as they are normally destroyed when the final labels are printed. No duplicate labels are printed when using this option.
Herb Labels: Corrected labels normally attached to a herbarium sheet. Final labels contain the herb code and accession number barcode at the bottom of the label. These labels should be printed on archive grade paper. Duplicate labels are printed according to the number of replicates specified in the "Replicate no.’ field of the unit screen. This allows labels to be printed without the need for knowing the destination of the specimen. Duplicate labels have the word ’Ex’ in front of the herbarium name at the top of the label. For each collection the printing order is the original label first followed by the specified number of duplicates.
Aust. Moss Packet: Moss labels for collections not flagged as foreign in the database. Duplicate labels are not printed using this option.
Ex. Aust. Moss Packet: Moss labels for collections flagged as foreign in the database. Duplicate labels are not printed using this option
Moss Duplicate: Used to print out duplicate labels for moss packets.
Box duplicate: No longer used.
Selecting the Labels:
Choosing any of the label types listed above from the menu opens the ‘Runtime Parameter Form’ in which you enter details of the labels you wish to print. Since labels are nothing more than a specially formatted report the ‘labels’ form is headed "Report Parameters’ and the name of the report to be printed appears in the title bar at the top of the form. This name is an abbreviated form of the label type you have chosen from the menu. The following fields are on the ‘Labels’ form.
Herbarium code. A drop down list allows selection of the herb code.
Accession No./Accession No last. These fields give a number of option for printing labels using the Accession number. Enter the number of the specimen for which you want to print the label.
-For a single label enter the accession number into the ‘Accession No’ field.
-For multiple labels where the accession numbers are sequential ( eg 640234,630235,630236) you can enter the first number into the ‘Accession No’ field and the last number in the sequence into the ‘Accession No. last’ field.
- For multiple labels where the accession numbers are NOT sequential you can enter the numbers into the ‘Accession No’ field with the numbers separated by a comma (eg 640234,642218,231459). Do not put any other form of punctuation in the field or put a period at the end of the sequence. Approximately 20 labels can be safely printed at a time using this procedure.
Note: Since the label form closes once the report is run (see below) it may be wise to construct your list in a text editor such as Notepad and then to paste it into the field. This will avoid having to retype the whole list in the event of a mistake being found after running the report. Hint-You can import lists of accession codes (or any other text) into the fields on this form by clicking in the desired field and selecting ‘Import text’ from the File menu. Choose the file you want to import and click OK. The contents of the file are copied into the selected field. The imported text must be in the correct format for the field into which it is copied or no labels will be generated.
Collector/Field Number/Field Number last. Labels can be printed using a combination of Collectors names and field numbers. These fields allow the same options as the ‘Accession No./Accession No last’ fields.
Note: The report will attempt to run even if you enter incomplete data. For example if you just type in a collectors name and no field numbers the system attempts to run a report on ALL collections by that collector. This may run to many thousands of collections and will almost certainly fail. This should be avoided.
Query clause. Labels may be generated using the equivalent of the ‘where’ clause used in SQL database queries. To use this mode you must be familiar with the field names used in the database. An example of this would be to leave ‘CANB’ in the ‘herbarium code’ field to type in ‘ACC_NO= 558345’ into the query clause field. This produces the same result as typing ‘558345’ into the ‘Accession number’ field. It returns all records that have a herb code ‘CANB’ and accession number ‘558345’.
Order by: You can choose the order that the labels are previewed and printed by changing the contents of this field. The default print order is by Herb code, Accession number and item_ID. You need to have a working knowledge of the database field names to order labels using other criteria.
Note: You can use the three buttons on the top left of the parameters form to cut/ copy and paste text from other sources or between fields on the form. To cancel the label printing click the red X button.
Previewing Labels: To run the report and see what the labels will look like when printed click on the green traffic light at the top of the form or hit Enter. This runs the report, closes the Reports Parameters form and opens the Preview form. This form should have the same label option displayed in the title bar as the previous form.
Labels are usually displayed 4 or 6 to a page depending on the amount of text on the label. Final labels are never more than 4 to a page. If there are more labels than can be displayed on a single page they are spread over the required number of pages and the first page is displayed. To view other pages use the arrow buttons at the top of the form. You can also zoom in and out to make the font size more readable.
Note: It is useful at this stage to check all the labels before printing. Often errors that were not obvious during data entry are visible at this stage. If errors are found then you can click the cancel button, fix the error and repeat the label creation process.
Printing Labels: Once you are satisfied that the information displayed on the labels is correct, print the labels using the button on the left of the button bar at the top f the form. This button should open the printer dialog box that will allow you to choose the correct printer and page set-up. Remember also to change the paper or paper source to print out on archive or recycled paper as required.
Geocoding
What is a geocode/Why calculate geocodes?
A geocode is a locality represented in a numeric (or alphanumeric) format. Whilst a written locality description is a vital information component of an herbarium specimen, a locality in geocode format is far more useful for plotting distribution maps, and generally investigating phytogeographic patterns. In this document, geocoding refers to calculating a numeric latitude and longitude from a written locality description. The following information will assist in the calculation of accurate, precise and meaningful geocodes.
Geocoding can be quite daunting initially, but the process quickly becomes easier. Your geographic awareness and specific knowledge of certain geographic areas will quickly develop, as will your familiarity with the geocoding process and geocoding resources. Initially, seek advice from colleagues who have more geocoding experience; and continue to do so when difficulties arise. It will always be useful to seek the advice of people who are more familiar with certain areas, for example, for a troublesome Tasmanian locality, consult a colleague who has lived in or visited Tasmania.
Which regions to geocode
All specimens collected from Australia and the Australian external territories need to be allocated a geocode as part of their data entry into ANHSIR (Australian National Herbarium Specimen Information Register). The only non-Australian specimens that are allocated a geocode, are those collected from Papuasia (roughly, the island of New Guinea, the Solomon Islands, and archipelagos in between). However, records from Papuasia are generally not geocoded at the initial ANHSIR data entry stage. They are geocoded from the ANHSIR record at some later stage. See a similar document to this 'The Papuasian Region and the Australian National Herbarium'.
The Australian external territories are Christmas Island (Ich), Cocos (Keeling) Islands (Ick), Ashmore & Cartier Islands (Ias), Coral Seas Islands (Ics), Norfolk Island (Ino), Heard & McDonald Islands (Ihe) and Australian Antarctic Territory (Aat). Lord Howe Island (Nlh) and Macquarie Island (Tma) are state administered islands (NSW and Tasmania respectively, hence the initial letter in their region codes) and require geocoding. For collections from all these non-mainland areas, record 'AUSTRALIA' in the ANHSIR political 'country' field, and apply the specific identifying three letter ANHSIR region code.
Geocode accuracy and precision
When calculating a geocode, use all the written locality information available to get as near as possible to the actual collection location. By using all of the information, the accuracy of the geocode is increased. Also, a measure of precision must be allocated to the geocode. The precision value indicates a distance from the calculated geocode, within which the actual collection locality occurs. A precision of 1000 m indicates that a circle of 1000 m radius, centred on the calculated geocode, will include the actual collection locality. It is important to note that the precision allocated to a geocode is as important as the geocode itself. The utility of a geocode, for a particular purpose, is indicated by the associated precision value.
If the written locality data could not produce a geocode accurate to less than around 150 km (e.g. if the collection locality is simply a state, or 'Inland Queensland', 'Central Australia' etc), it is probably not worth calculating a geocode, as a distribution 'point' on a map representing the specimen could be vastly different to the actual collection locality. This could potentially place the specimen outside of its normal distributional range, and in a completely different habitat. To calculate a geocode in such cases is more likely to be misleading than to provide meaningful information.
It is not worth geocoding an Australian specimen where the locality is only traceable to one of the six states or the Northern Territory. It is worth geocoding a specimen where the locality is only traceable to the ACT, Jervis Bay Territory or one of the external island territories.
NOTE: the precision code system, previously used by ANH, was merely used as shorthand to generate a ballpark precision value in metres. The actual code was not important, it was the precision value in metres that was desired. Whilst these codes are still available, do not limit yourself to these ballpark precisions. Instead, you are encouraged to allocate a precision value in metres. By doing so, the geocodes in ANHSIR will be far more precise, especially for geocodes with a precision that is not well represented by the code system. For example, in the case of a geocode accurate to 3 km, it is far more meaningful to enter an accuracy of 3000 m directly into the 'in metres' field, than allocate the precision code of 3 (which would be required if relying on the codes), that will result in a generated precision of 10 000 m. The same geocode, deemed precise to within 3 km, is far more useful than one only deemed precise to within 10 km. In this example we have increased the utility of the calculated geocode immensely, by entering a value in metres, rather than relying on the code system. In fact the size of the 'on ground' precision land parcel, when 3 km is denoted, is less than one tenth of the area associated with a 10 km precision.
NOTE: The Geocode Ready Reckoner provided at the end of this document may be useful when allocating precision. It gives an indication of the on ground distance represented by different latitude measurements and units. Theyare listed in appendix 3.
The following table provides a target rule of thumb as to how precisely metre precision values should be calculated and recorded (rounded). The metre precision values equate to roughly 10% of the geocode precision range.
Geocode precision value falls within: |
Calculate precision value to the nearest: |
50–1000 m |
50–100 m |
1–10 km |
0.5–1 km |
10–20 km |
1–2 km |
20–100 km |
2–10 km |
> 100 kms |
10–25 km |
For example, it is not worth putting in the excessive effort to calculate a precision figure of say 14.7 km. There is always an element of guesstimating in the geocoding process and rather, than attempt to be pedantically and imaginarily accurate and quote 14.7 km, simplify the process and think in terms of whole kilometres. In this case, record 15 km. Similarly it is probably excessive effort to try to calculate and record a precision of 38 km. It is much easier and practically as useful, to simply think in blocks of 5 km, and record 40 km.
Even with relatively imprecise localities, calculate a geocode to the nearest minute. This is a far more accurate approach than to calculate a geocode only to degrees, or to round to the nearest 5 or 10 minutes. Also, if a geocode on a label is only quoted to degrees, or to only the first digit of the minutes, please calculate a geocode to the nearest minute.
Geocoding resources
Two of the most useful resources when geocoding Australian specimens are:
- Geoscience Australia Place Names Search: www.ga.gov.au/map/names/, and
- Readers Digest Atlas of Australia.
Other resources include:
- Australia Unfolded, an interactive CD-ROM atlas.
- Microsoft Encarta - a world atlas that can be installed on your PC.
- Decimal Latitude/Longitude converter: www.geology.enr.state.nc.us/gis/latlon.html
- Grid Reference to Latitude/Longitude converter: www.ga.gov.au/nmd/geodesy/datums/redfearn_grid_to_geo.jsp
- Hard copy 'Gazetteer of Australia' book.
- On your PC: P-Drive - ANH - ANH_Resources - Australian Extra Localities - an in house compilation WORD file (a last resort option).
If you have a need to geocode Papuasian specimens, please refer to the document 'The Papuasian Region and the Australian National Herbarium'.
An excellent website explaining geocoding concepts in detail, can be found at the MaNIS (The Mammal Networked Information System) website, Georeferencing Guidelines page: http://elib.cs.berkeley.edu/manis/GeorefGuide.html
It will give you a good background on what can be reliably interpreted from a written locality description, and some basic geometry concepts that are important. Well worth a look.
Always use standard geocodes where they are available, eg
- CSIRO Black Mountain (glasshouses): 35 deg. 16 min. S, 149 deg. 7 min. E; precision 1000 m (code 2).
- Australian National Botanic Gardens: 35 deg. 17 min. S, 149 deg. 7 min. E; precision 1000 m (code 2).
For a frequently encountered locality where you want to get a precise latitude/longitude, there are many detailed maps available in the map room. It is time consuming to go to this trouble for a geocode, so if you do, please add the geocode to one of the in-house gazetteers where it is available for others to use. A locality example that may justify such time input may be a research station or a botanic garden.
For locations that are not traceable through the above resources, try querying ANHSIR. There may be another collection from the same locality already databased and geocoded. This is particularly useful for localities which have been misspelt on labels. Often, the correct spelling has been included with the record, in square brackets. Please perpetuate this system of including the correct spelling of place names, for records where there is an incorrect spelling on the label.
When all else fails an internet search may be useful.
You may need to use a combination of resources to be confident you have selected the correct locality, particularly when there is some ambiguity as to which, for example, Black Mountain, is being referred to. Particularly in Queensland, you often have to use the botanical region (if it has been provided) to resolve ambiguous localities.
If you are unable to geocode a specimen, please let me know, and I will attempt it. There may be a technique you are unaware of. There will of course always be localities that are not traceable.
Geocoding hints
A common barrier to calculating geocodes is interpreting the herbarium label, especially when it is handwritten. If you are having trouble reading a label, ask someone for a second interpretation, they'll often instantly read the letters differently to your interpretation.
Sometimes labels have incorrectly spelt place names, especially where double letters are involved; there may be double letters, when there should only be one, or vice versa, ie Amaroo and Ammaroo. Having a superfluous 's', or missing an 's' is another common problem. Names like Williams Creek may actually be William Creek. Some localities may have two words contracted into one, or vice versa. If a label says Middlecove, try Middle Cove.
The above William Creek example, shows the advantage of not entering the entire place name into an electronic gazetteer. Instead of typing the entire 'Williams Creek'(which would not return William Creek) use wildcards and type 'Will% Cre%'. The search may be a little slower and may bring back more results (it would bring back, for example, Willford Crematorium, if such a place existed in the gazetteer) but at least you won't miss out on the target, William Creek.
When using gazetteers, places such as Mount (Mt) Wellington may need to be searched for under Wellington rather than Mount (or Mt). Think similarly for places like Lake Argyle and Wallaga Lake. The collector may have recorded the words in the wrong order.
Think about localities such as 'Port Melbourne' (found on some old collections) and 'Sydney Harbour'. A geocode for these locations from a gazetteer will give you a point in the water. Most specimens we deal with are terrestrial! Try to find a point around the body of water and then allocate a suitable precision value (unless the plant really is aquatic). Think similarly for inland water features. Whilst technically speaking, a distribution dot in a body of water with suitable precision incorporating terrestrial habit is not incorrect, it is clearly not the collection locality, and therefore should be avoided.
The age of a label often needs to be taken into account. Old labels (say, pre early to mid 1900's) simply saying, for example, 'Dubbo', need to be tackled with caution. Examples such as these should probably be read as 'Dubbo area' and probably need a precision of around 30 km. Labels saying '80 km west of Esperance' probably mean 80 km west by road, and presumably by the main road. We assume distances to be land distances (not as the crow flies, unless the label says 'direct' or 'by air'), and assume them to be by a road, unless there is a reason to believe otherwise.
Populating the Geocode Related ANHSIR Fields
A geocode and a precision value need to be entered into ANHSIR for most specimens. It is a fairly intuitive process to enter the geocode into the separate degree, minute and second fields. Ensure that the appropriate compass direction is indicated in the direction fields. By default a new record is given compass directions of 'S' and 'E', which are appropriate for Australia. This will need to be changed for many non-Australian specimens. Often old existing ANHSIR records have the direction fields blank. Please enter the appropriate direction.
If a calculated geocode is 35 degrees and 0 minutes, always enter '0' in the minutes field, don't leave the field blank, as technically, a geocode with blank 'minutes' is not as accurate as a geocode with '0' 'minutes'. If there are no seconds recorded for a geocode, it is correct to leave the field blank.
The method used to obtain the geocode, and the geocode source, need to be entered into ANHSIR.
Geocode Method:
(a) Only use 'GPS' (Global Positioning System) for geocodes where it is stated on the label that a GPS has been used. Such geocodes are almost always quoted to the nearest second, but may be in a decimal form (eg 23.6587 deg S or 23 deg 25.368 min. S). GPS readings can be allocated a precision of 50 m (code 1). Occasionally a geocode includes a decimal point of a second. In such cases, round to the nearest second to populate the 'second' field, but include the full geocode in the 'locality' field (this can simply be written '35 17 16.4 S, 149 6 58.7 E').
(b) 'Generalised arbitrary point' can be used when a midpoint between two localities is calculated, where you cannot trace the fine locality details provided on the label, or if you feel the collector only provided a generalised locality - particularly for older collections.
(c) 'Site located on map' should be selected if you have traced a locality on a map and read off a geocode, or if you have used a gazetteer.
(d) 'Vector from a named place' is not commonly used. However, if you have traced a collection locality on a map in this manner (eg, 10 km SW of Braidwood, and there is no apparent road in this direction), or if the label says, for example '13 km E of Mt Isa by air', select this option. Labels that say, for example, 10 km directly SE of Braidwood, imply 10 km via a vector on a map, rather than via a road.
(e) Select 'Unknown' when you aren't sure how a geocode has been calculated, which will be the case for most specimens from other institutions, where a geocode is printed on their label.
It should be fairly intuitive to select the appropriate geocode source from the options in ANHSIR.
In some unusual geocoding cases you may wish to add a comment in square brackets in the locality field, indicating how the geocode was calculated. For example [geocode is that of Tilba Tilba] where you were unable to trace a specific locality description, that mentions Tilba Tilba. You should enter a comment in square brackets if you have chosen not to calculate a geocode, to indicate that calculating a geocode has not been overlooked; [insufficient locality details for a useful geocode] would be appropriate. Where a geocode has not been calculated, there is no need to enter anything in the geocode 'precision', 'method' or 'source' fields, as there is no geocode to relate to these geocode attributes.
It can be difficult to decipher an appropriate precision code for specimens geocoded by other organisations. Use the geocode provided by the other institution. In terms of the precision value, it may be best to consider the written locality description provided, and estimate how precise a geocode you would be able to calculate, if one hadn't been provided.
It is important to use 'local knowledge' when considering how to populate the geocode related fields; eg C.W.E. Moore always allocated geocodes to his own collections, A. Fraser calculates her geocodes from a map and gives distances by air, and AD (State Herbarium of South Australia) often allocate a specimen the geocode of the nearest named place, not of the collection locality.
If a grid reference is supplied on the label, it should be entered verbatim into 'geo grid' field. Rarely is a grid reference supplied in the correct format, or in full; but a complete grid reference can be entered into a converter programme (see Geocoding resources) to produce a latitude and longitude.
Datum is not something that you need to calculate or look up. It will either be recorded on a label or it won't. A datum is basically a model of the earths surface, that a coordinate system can be applied to (we use latitude/longitude as the coordinate system). If you are keen to understand datums, http://www.ga.gov.au/nmd/geodesy/datums/ The four most likely datum candidates that may appear on a label are: GDA94, MGA94, AGD84, AGD66.
The first three letters are an acronym of the model, and the two following numbers refer to a year of the 1900's that the datum was calculated.
Troubleshooting
Advanced querying options
Most records in ANHSIR can be retrieved by querying the herbarium code and accession number on either the LABEL screen or the EVENT screen. However, there will be times when neither of these query options appear to work, even though you know the record has been entered, or have a strong reason to suspect that the record has be entered. When this occurs there are a couple of slightly more advanced querying options which can be helpful. The first is to query for information other than the herbarium code and accession number on the LABEL screen. For example, you might try a combination of the collector and field number, or collector and date. However, if the reason that the record is not being retrieved is that it does not have a determination flagged as being current, no LABEL screen query will be successful in retrieving the record.
If LABEL screen queries are unsuccessful there are some variations on the EVENT screen query which may help (*Remember that for any EVENT screen query to be successful the LABEL screen must be blank). Try querying the EVENT screen using the collector name and some details from the locality field. Or, if you were the person who entered the record you may choose to query by compiler. To do this, press F7 to query, change from "Georeference" to "Meta" and enter your user name, in capitals (e.g. LHALASZ), and the date the record was entered (e.g. 10-NOV-2002). If you are unsure of the exact date it is possible to use a wildcard (%) here. Press F8 and all the records you entered on that date should be returned, including any unfinished records.
Appendix 1: Function Keys
Most commonly used function keys:
Accept/Save – F10
Cancel – Esc
Copy field – F3
Copy screen – F4
Delete record – Shift F6
Enter Query – F7
Execute Query – F8
Exit – Ctrl q
Help – F1
Insert Record – F6
List of Values (lookup table) – F9
Next field – Tab
Next screen – Ctrl Page Down
Previous field – Shift Tab
Previous screen – Ctrl Page Up
NOTE: by pressing F1 anywhere within in the database and selecting "keys" a full list of the available function keys will be displayed.
NOTE: there are field specific hints located throughout the database. These hints are displayed across the bottom of the screen and related to whichever field the cursor is currently located in.
Appendix 2: Useful web addresses
Unit Converter - digitaldutch.com/unitconverter/
Merriam-Webster online Dictionary - www.m-w.com
Foreign language translator - wordreference.com
Index Herbariorum - www.nybg.org/bsci/ih/ih.html
ANHSIR online Query - www.anbg.gov.au/cgi-bin/anhsir
Worldwide Directory of Cities and Towns - www.calle.com/world/
Global Gazetteer - gnpswww.nima.mil/geonames/GNS/index.jsp
Geoscience Australia (formerly Auslig) Online Gazetteer - www.auslig.gov.au/mapping/names/natgaz.htm
International Plant Name Index - www.uk.ipni.org
Australian Plant Name Index - www.anbg.gov.au/cgi-bin/apni
Decimal lat long Converter - www.geology.enr.state.nc.us/gis/latlon.html
Search Engines:
Google - http://www.google.com
GoEureka - http://www.goeureka.com.au
MetaCrawler - http://www.metacrawler.com
AltaVista - http://www.altavista.com
Vivismo - http://vivisimo.com/
Appendix 3: Precision Codes/Source codes
Precision codes:
1 – within 50 m or 1"
2 – within 1 km or 1’
3 – within 10 km or 5’
4 – within 25 km or 10’
5 – greater than 25 km or 30’
6 – indefinable within the Australian Continent
Source codes:
1 – collector
2 – compiler
3 – automatically generated
4 – records of another institution
5 – unknown
Herb material codes:
H – herbarium sheet
A – specimen stored in alcohol
F – fruit separate
K – floral card
V – vertical packet (cryptogam)
B – box (cryptogam)
W – wood sample
Gardens material codes:
P – plant
C – cuttings
S – seed
L – unspecified living material
Appendix 4: Geocode Ready Reckoner
Distance |
|
Latitude Arc |
|
3.1 |
m |
0.1 |
sec |
31 |
m |
1 |
sec |
50 |
m |
1.61 |
sec |
100 |
m |
3.23 |
sec |
200 |
m |
6.45 |
sec |
250 |
m |
8.07 |
sec |
310 |
m |
10 |
sec |
500 |
m |
16.23 |
sec |
930 |
m |
30 |
sec |
1 |
km |
32.26 |
sec |
1.86 |
km |
1 |
min |
2 |
km |
1.06 |
min |
5 |
km |
2.66 |
min |
10 |
km |
5.31 |
min |
11.13 |
km |
0.1 |
deg |
18.53 |
km |
10 |
min |
20 |
km |
10.63 |
min |
25 |
km |
13.25 |
min |
37.06 |
km |
20 |
min |
50 |
km |
26.57 |
min |
55.56 |
km |
30 |
min |
100 |
km |
53.13 |
min |
111.33 |
km |
1 |
deg |
200 |
km |
1.8 |
deg |
250 |
km |
2.25 |
deg |
500 |
km |
4.49 |
deg |
1000 |
km |
8.98 |
deg |
1112 |
km |
10 |
deg |
NOTE: measurements calculated from degrees of latitude around Canberra, Sydney.
Appendix 5: Donor Institutes list
# Code |
Prefered Entry |
Donor type |
Real Name |
Comments |
#BEAU |
A.C.Beauglehole |
Individual |
|
|
#ACHAP |
A.D.Chapman |
Individual |
|
|
#KANIS |
A.Kanis |
Individual |
|
|
#ANDREWS |
A.LeRoy Andrews |
Individual |
|
|
|
A.Morrison |
Individual |
|
|
#VEZDA |
A.Vezda |
Individual |
|
|
#ARCHER |
A.W.Archer |
Individual |
|
|
#ACTPC |
ACT Parks and Conservation |
Other |
|
|
#ANTARC |
ADT |
Herbarium |
Kingston: Herbarium, Antartic Division, Kingston, Tasmania, Australia. |
|
#ABRS |
Australian Biological Resources Study |
Other |
|
|
#ANU |
Australian National University |
University |
|
|
|
Australian School of Forestry |
Other |
|
|
|
Australian Tree Seed Centre |
Other |
|
Part of FRI |
#WHITE |
B.Whitehead |
Individual |
|
|
|
BISH |
Herbarium |
Honolulu: Herbarium, Botany Department, Bishop Museum, Honolulu, Hawaii, U.S.A. |
|
|
BK |
Herbarium |
Bangkok: Herbarium, Botany Section, Botany and Weed Science Division, Department of Agriculture, Bangkok 10900, Thailand. |
|
#BRISA |
Botanical Research Institute South Africa |
Other |
|
|
#BELGIUM |
BR |
Herbarium |
Meise: Herbarium, Nationale Plantentuin van Belgie, Jardin Botanique National de Belgique, Domein van Bouchout, Meise, Belgium. |
|
#BFC |
Bulolo Forestry College Herbarium Papua New Guinea |
University |
|
|
#BFF |
Bureau of Flora and Fauna |
Unknown |
|
|
#INGR |
C.K.Ingram |
Individual |
|
|
#LUNDELL |
C.L.Lundell |
Individual |
|
|
#CANTU |
CANU |
Herbarium |
Christchurch: Herbarium, Department of Plant and Microbial Sciences, University of Canterbury, Christchurch I, New Zealand. |
|
#CIMB |
Centro de Investifation de Biologia Marina Argentina |
Research, field or experiment station |
|
|
|
Cleveland High School Seattle Washington USA |
Other |
|
|
#ANIC |
CSIRO Australian National Insect Collection |
Other |
|
|
|
CSIRO Division of Wildlife Research |
Other |
|
Note: the CSIRO Division of Wildlife Research was active between 1962 and 1982, collections from after 1982 should be attributed to CSIRO Wildlife and Ecology Canberra |
|
CSIRO Division of Wildlife Research Perth |
Other |
|
|
#RPLA |
CSIRO Regional Pastoral Laboratory Armidale |
Research, field or experiment station |
|
|
CSIRO WARL |
CSIRO Western Australian Regional Laboratory |
Research, field or experiment station |
|
|
|
CSIRO Wildlife and Ecology Canberra |
Other |
|
|
#RATKOW |
D.A.and A.V.Ratkowsky |
Individual |
|
|
#CATCH |
D.G.Catcheside |
Individual |
|
|
#HERBST |
D.Herbst |
Individual |
|
|
|
Dames and Moore private consultant NSW |
Other |
|
Distributed by Dames and Moore, Crows Nest, NSW. |
#ALOTAU |
Department of Forests Alotau Papua New Guinea |
Other |
|
|
|
Department of Sustainability & Environment, Vic |
Nursery |
|
|
#KUNUNURRA |
Dept of Cons. and Land Management Kununurra |
Other |
|
|
#NRH |
Division of Cons Parks and Wildlife Nrn Reg Herb |
Other |
|
|
#LUMBSCH |
E. and H.T.Lumbsch |
Individual |
|
|
#DAHL |
E.Dahl |
Individual |
|
|
#MG |
E.Goeldi |
Individual |
Museu Paraense Emilio Goeldi |
|
#MCBARR |
E.J.McBarron |
Individual |
|
|
#EMU |
EMC |
Herbarium |
Ypsilanti: Herbarium, Biology Department, Eastern Michigan University, Ypsilanti, Michigan, U.S.A. |
|
#ESSEN |
ESS |
Herbarium |
Essen: Herbarium, Fachbereich 9, Botanik, Universitat Essen-GHS, Postfach, Essen I, Federal Republic of Germany. |
|
#ENBG |
Eurobodalla Native Botanic Gardens |
Botanic Garden |
|
|
#CAIRNS |
Flecker Herbarium |
Other |
|
The Flecker Herbarium of the North Queensland Naturalist's Club was transferred to QRS in 1971; Bryophytes were transerred to CANB in 1981. Ref: Flora of Australia, vol. 1. |
#FTB |
Forestry and Timber Bureau Atherton QLD |
Other |
|
|
#FTBC |
Forestry and Timber Bureau Canberra |
Herbarium |
|
|
#FTB-C |
Forestry and Timber Bureau Canberra |
Herbarium |
|
|
#FCOM |
Forestry Commission of NSW |
Other |
|
|
|
FRI |
Herbarium |
|
NOTE: Any specimen which has an FRI accession number should be coded as FRI. The actual name of the donor at the time should be added to the comment field, e.g. Forestry and Timber Bureau, Canberra; Australian School of Forestry etc |
|
G.Beaton |
Individual |
|
|
#BOTSNM |
GAB |
Herbarium |
Gaborone: National Herbarium, National Museum, Monuments, and Art Gallery, Gaborone, Botswana. |
|
#MILLER |
H.A.Miller |
Individual |
|
|
|
H.B.Williamson |
Individual |
|
|
#HURLIMANN |
H.Hurlimann |
Individual |
|
|
#ULLRIC |
H.Ullrich |
Individual |
|
|
#RWTH |
HAQ |
Herbarium |
Aachen: Herbarium, Fachbereich Biologie, Botanisches Institut der Techn. Hochschule (RWTH), Postfach, Aachen, Federal Republic of Germany. |
|
#HPEL |
Herbarium Plant Ecology Laboratory |
Unknown |
|
|
#EICHLER |
Hj.Eichler |
Individual |
|
|
#CAXIAS |
HUCS |
Herbarium |
Universiadade de Caxias do Sul |
|
#MATTH |
I.G.Matthias |
Individual |
|
|
|
I.V.Newman |
Individual |
|
|
#ELIX |
J.A.Elix |
Individual |
|
|
#BEEVER |
J.E.Beever |
Individual |
|
|
#HAFELLNER |
J.Hafellner |
Individual |
|
|
#HEINRICHS |
J.Heinrichs |
Individual |
|
|
#SLOOVER |
J.L.De Sloover |
Individual |
|
|
#MUNOZ |
J.Munoz |
Individual |
|
|
#FRAHM |
J.-P.Frahm |
Individual |
|
|
#CROFT |
J.R.Croft |
Individual |
|
|
#KALB |
K.Kalb |
Individual |
|
|
#THIELE |
K.R.Thiele |
Individual |
|
|
#KMX |
Karl Marx Universitat Germany |
University |
|
|
|
Kings Park Botanic Garden |
Botanic Garden |
|
|
#KOCH |
Kochi Gakuen College Japan |
University |
|
|
KOS |
Kosciusko State Park Herbarium |
Other |
|
|
#KUMA |
KUMA |
Herbarium |
Kumamoto: Herbarium, Biology Department, Faculty of Scinece, Kumamoto University, Kurokami 2-chome, Kumamoto-shi, Kimamoto, Japan. |
|
#LICADY |
L.I.Cady |
Individual |
|
|
#PNGUT |
L.J.Brass Memorial Herbarium PNG Uni of Technology |
University |
|
|
|
LIL |
Herbarium |
San Miguel De Tucuman: Herbario, Area Botanica, Fundacion Miguel Lillo, Miguel Lillo 251, 4000 San Miguel de Tucuman, Tucuman, Argentina |
|
#HMC |
M.D.Crisp |
Individual |
|
|
#MAHU |
M.Mahu |
Individual |
|
|
#SEAWARD |
M.R.D.Seaward |
Individual |
|
|
#MSC |
M.S.Christiansen |
Individual |
|
|
#MACU |
Macquarie University |
University |
|
|
MAH |
MAH |
Herbarium |
Mahalapye: Herbarium, Department of Agriculture, Mahalapye, Botswana - transferred to GAB. |
|
|
Meekatharra District Office Herbarium |
Other |
|
|
|
MSC |
Herbarium |
East Lansing: Michigan State University Herbarium |
|
#MURCIA |
MUB |
Herbarium |
Murcia: Herbario, Departamento de Biologia Vegetal (Botanica), Eniversidad de Murcia, Murcia, Spain. |
|
#MAASH |
Museum of Applied Arts and Sciences Herb. Sydney |
Other |
|
|
|
N.H.Sinnott |
Individual |
|
|
#NPAV |
National Parks Authority VIC |
Other |
|
|
#NYSED |
New York State Education Department |
Municipal Department |
|
|
#NZFC |
New Zealand Department of Forestry College |
Other |
|
|
NAQS |
North Australian Quarantine Survey Mareeba |
Other |
|
|
#NCALM |
NSW Department of Cons and Land Management |
Other |
|
|
#NNPWS |
NSW National Parks and Wildlife Service |
Other |
|
|
#NPWS |
NSW National Parks and Wildlife Service |
Other |
|
|
#FCNSW |
NSWF |
Herbarium |
Sydney: Herbarium, Wood Technology and Forest Research Division, Forestry Commission of New South Wales, Beecroft, New South Wales, Australia. |
|
#DEGENER |
O.Degener |
Individual |
|
|
|
O.W.Borrell |
Individual |
|
|
|
Oklahoma Agricultural and Mechanical College |
Other |
|
|
#OMAN |
ON |
Herbarium |
Muscat: National Herbarium of Oman, Natural History Museum, Ministry of National Heritage and Culture, Muscat, Oman. |
|
#GEISSLER |
P.Geissler |
Individual |
|
|
#PRDNZ |
Parks and Recreation Department New Zealand |
Other |
|
|
#PILBARA |
Pilbara Regional Herbarium |
Other |
|
|
#UNITECH |
PNG University of Technology |
University |
|
|
#CZECH |
PRA |
Herbarium |
Czechoslovakia Acadamy of Sciences, Czechoslovakia |
|
#PRETORIA |
PRE |
Herbarium |
|
|
#QNPWS |
QLD National Parks and Wildlife Service |
Other |
|
|
#HOOGLAND |
R.D.Hoogland |
Individual |
|
|
#SCHOM |
R.D.Schomburgk |
Individual |
|
|
#RDS |
R.D.Seppelt |
Individual |
|
|
#SEPPELT |
R.D.Seppelt |
Individual |
|
|
#REA |
REA Hortus-Botanicus Tranensis Herbarium |
Unknown |
|
|
|
RSA |
Herbarium |
Claremont: Herbarium, Rancho Santa Ana Botanic Garden, California, U.S.A. |
|
#BLAKE |
S.T.Blake |
Individual |
|
|
#SCHAEFER |
Schaefer-Verwimp |
Individual |
|
|
#SOH |
School of Horticulture |
Other |
|
|
#MSBGH |
SEL |
Herbarium |
Sarasota: Herbarium, Marie Selby Botanical Gardens, Sarasota, Florida, U.S.A. |
|
#SMHA |
Snowy Mountains Hydro-electric Authority |
Other |
|
|
#SCSC |
Soil Conservation Service of NSW Cooma |
Other |
|
|
#SCSW |
Soil Conservation Service of NSW Wagga Wagga |
Other |
|
|
#CHILE |
SQF |
Herbarium |
Santiago: Herbario, Escuela de Quimica y Farmacia, Laboratorio de Botanica, Facultad de Ciencias Quimicas y Farmaceuticas, Universidad de Chile, Santiago I, Chile. |
|
#FIJI |
SUVA |
Herbarium |
Suva: South Pacific Regional Herbarium, School of Pure and Applied Sciences, University of the South Pacific, Suva, Fiji. |
|
#SAUS |
Swedish University of Agricultural Sciences |
University |
Uppsala: Sweden |
|
|
T.B.Paltridge |
Individual |
|
|
#REEVE |
T.M.Reeve |
Individual |
|
|
#TARTU |
TAA |
Herbarium |
Tartu: Herbarium, Institute of Zoology and Botany, Tartu, Estonian S.S.R., U.S.S.R. |
|
TARCH |
Tamworth Agricultural Research Centre Herbarium |
Research, field or experiment station |
|
|
#TOTT |
Tottori University Japan |
University |
|
|
#TUNG |
TUNG |
Herbarium |
Taichung: Herbarium, Biology Department, Tunghai Univeristy, Taichung, Taiwan, Republic of China. |
|
#UGDA |
UGDA |
Herbarium |
Gdynia: Herbarium, Department of Plant Ecology and Nature Protection, Gdansk University, Czolgistow, Gdynia, Poland. |
|
|
UMO |
Herbarium |
Colombia: Herbarium, Biological Sciences Division, University of Missouri, Columbia, Missouri, U.S.A. |
|
#NITAB |
University of Technology Sydney |
University |
|
Formerly NSW Institute of Technology, Department of Applied Biology. |
#NSWUT |
University of Technology Sydney |
University |
|
Formerly NSW Institute of Technology, Department of Applied Biology. |
#UNIWOL |
University of Wollongong |
University |
|
|
#USDA |
US Department of Agriculture |
Other |
|
|
|
W.H.Ewers |
Individual |
|
|
#HOE |
W.J.Hoe |
Individual |
|
|
#WAWRC |
WA Wildlife Research Centre |
Other |
|
|
#WEI |
WAU |
Herbarium |
Lae: Herbarium, Wau Ecology Institute, Wau, Morobe, Papua New Guinea. |
|
#WOTAFE |
Woden College of TAFE |
Other |
|
|
#WOOL |
Woolcock |
Individual |
|
|
Appendix 6: Abbreviations and Expansions
We generally duplicate the label when databasing, however, if a label contains abbreviations, then the following abbreviations should be used. For example, if a label states "Mount Thomas" we will type "Mount Thomas", but if the label chooses to abbreviate "Mount" then we will always use the form "Mt", not "Mt." or "Mnt"
Mount
Mt
km (kilometres) NOTE: always leave a space between number and units e.g. 12 km, not 12km
cm (centimetres)
m (metres)
m. or mi.(miles)
ft (feet)
S (south)
N (north)
E (east)
W (west)
sp. (species)
subsp. (subspecies)
spp. (multiple species e.g. Hakea spp. = more than one species of Hakea)
var. (variety)
f. (form)
c. (circa – anywhere in a sentence)
Ca (circa – at the beginning of a sentence)
ca (circa – in the middle of a sentence)
rd (road)
Expansions
The following expansions should be made when databasing:
NP – National Park
SP – State Park
CP – Conservation Park
SF – State Forest
FR – Forest Reserve
CR – Conservation Reserve (SA in particular)
HS – Homestead
QRS abbreviations, which should be expanded in square brackets
S.F.R. – State Forest Reserve
Cpt – Compartment
N.P.R. – National Park Reserve
L.A. – Logging Area
T.R. – Timber Reserve
V.C.L. – Vacant Crown Land
Por. – Portion
Frequently asked questions:
Q: I know that this specimen is databased, but I can’t seem to retrieve the record from a LABEL screen query?
A: If there is no determination for the record, or none of the determinations is flagged as being current, the record will not be retrieved from a LABEL screen query. To retrieve the record try querying from the EVENT screen instead (but remember to add a det or flag an existing det as being current before leaving the record again!).
Q: I know that this specimen is databased, but I can’t seem to retrieve the record from an EVENT screen query?
A: To successfully query from the EVENT screen it is essential that the LABEL screen is blank. Check your LABEL screen, clear it if necessary, and try again.
Q: What should I do if there is no collector identified for the specimen I’m databasing?
A: Since the collector field must be filled in to proceed, we use the latin abbreviation "leg.ign." to indicate that the collector is unknown.
Q: What should I do if there is no field number shown on the specimen I’m databasing?
A: It is important that we don’t leave the field number field blank, so where there is not number given we use the latin abbreviation "s.n." to indicate that the collection has no number.
Q: Why do I have the option of entering the "field number" on the EVENT screen as well as the UNIT screen?
A: If the field number is entered at the EVENT screen level the database has a chance to check whether or not that particular combination of Collector and field number already exists in the database. This can be useful for identifying whether a version if this collection has already been databased, for example, if there is another sheet of the same collection already on the system.
Q: Do I need to enter the "point" associated with the accession number on the ITEM screen?
A: No. The "points" .1, .2, .3 etc are generated automatically as a way of identifying unique ITEMs of collections which have multiple ITEMs with the same accession number. Currently it is standard practice to allocate only one accession number per collection, which means that several ITEMs/sheets may have the same accession number.
NOTE: It was not always standard practice to have the same accession number for different ITEMs/sheets of a collection, and it is possible for such ITEMs to have different accession numbers, however it is worth noting that the "points" will still be automatically allocated.
Q: What is a dummy sheet?
A: A dummy sheet is a marker in the collection. For example, type specimens are not housed in the general collection, they are keep in a separate Type Room where they are protected from fire and over-handling. There is a dummy sheet in the collection for each specimen in the Type Room, they serve to indicate to anyone using the collection that there is a Type specimen housed in another location that they might be interested in looking at. Dummy sheets are used as markers for Type specimens, specimens in alcohol/spirit, and floral cards.
Q: Why would 2 sheets of the same collection end up with different determination histories?
A: Often when herbaria close down, or individuals collections are donated, previously separated duplicates of the same collection are brought back together. It is usually the case in these instances that as a result of being housed in different locations and curated by different people that these two sheets will have different determination histories. Even if the determinations are the same, the person making the determination is usually different.
Q: Sometimes I need to query the database for information, but when I do I loose all the records I’ve entered during that session. How can I query but still keep those records?
A: It can be very useful to have all of the records entered in a particular session available on the screen for reference or editing. The best way to query for other information is to have two versions of ANHSIR open on the desktop at the same time. This also enables you to cut and paste from the queried record to the one being entered.
Q: Sometimes when I enter collections with two sheets they have the same herb code and accession number and sometimes they are different. Why is that?
A: The current Australian National Herbarium (ANH) contains specimens from both CANB and CBG, and prior to their amalgamation these two herbaria used different accessioning systems. The protocol at CANB was to allocate a unique herbarium code and accession number combination to every sheet in a collection. The protocol at CBG was to allocate a unique herbarium code and accession number to each collection, regardless of the number of sheets it contained. To cope with these two methods ANHSIR employs a system of "points". Any ITEM that is saved in the database is allocated a point e.g. .1, .2, .3. If there are two sheets with the same accession number they will be distinguished by their unique points e.g. 628148.1 and 628148.2. If the accession numbers are different, they are still allocated points, even though it is unnecessary e.g. 628148.1 and 628149.2
NOTE: at the time of dataentry the points should be added to the herbarium sheets where the accession numbers are the same. It is advisable to use an archival pen for this purpose.
![An Australian Government Initiative [logo]](/images/austgovt_canbr_90px.gif)




