Part of a Sowerby painting, labelled as Lichen digitatus but the species shown here is now in the genus Cladonia. There are a number of Cladonias with red apothecia, both in Australia and overseas, and in all cases the red pigment is rhodocladonic acid. |
Chemistry
As a group, lichens are rich in chemical compounds and even anyone with no chemistry background can see evidence of this fact. Just look at the array of colours you find in lichens - various greens, yellows, reds, oranges and browns as well as shades of white and grey and there are even black lichens. These pigments are examples of some of the chemical compounds present in lichens but there are far more colourless compounds present and these need more than the naked eye for detection![]() .
.
The compounds produced by lichens fall into two classes: the primary (or intracellular) metabolites and the secondary (or extracellular) metabolites. Various printed or web sources use the expressions lichen acids or lichen substances instead of the term secondary metabolites. Amino acids, proteins, vitamins and polysaccharides are examples of primary metabolites and the majority of primary metabolites are not specific to lichens but are also found in non-lichenised fungi or other organisms. Amongst the primary metabolites some are produced by the fungus and some by the photobiont but it is not always easy to determine which partner is responsible for a particular compound. The secondary metabolites are all produced by the fungus which utilizes the carbohydrates it gains from the photobiont. While the secondary metabolites are initially produced within the fungal hyphae they are finally deposited on the hyphal surfaces, either as crystals or as amorphous deposits. Many of the primary metabolites are soluble in water and can be extracted in boiling water but the secondary metabolites are generally insoluble in water and must be extracted with organic solvents. Over 700 secondary metabolites are known and the great majority have been found only in lichens, with just 50 to 60 of them found also in non-lichenized fungi or in the plant kingdom. While lichens as a whole are rich in secondary metabolites the richness is not uniform across all groups of lichens. Those with cyanobacterial photobionts are generally poor in secondary metabolites as are certain crustose groups.
The photobiont is the source of carbohydrate for the lichen via photosynthesis and the type of carbohydrate provided to the mycobiont depends on the photobiont. Green algae supply polyhydric sugar alcohols (or polyols) while cyanobacteria provide glucose. Examples of polyols supplied by green algal photobionts are ribitol (supplied by photobionts in the genera Coccomyxa, Myrmecia and Trebouxia), erythritol (supplied by Trentepohlia) and sorbitol (from Hyalococcus). Once harvested by the fungus the carbohydrate is irreversibly metabolized into a form unusable by the photobiont![]() .
.
Of the 700 plus secondary metabolites the great majority are found on medullary hyphae with relatively few in the cortices. Many of the lichens with secondary metabolites contain just a few such compounds, commonly with one metabolite produced in the cortex and one to a few in the medullary hyphae. In certain species some metabolites are quite restricted, for example, found only in the apothecia or in the soredia. While many lichens produce only a few secondary metabolites there are the striking exceptions, such as a number of species in the genus Pseudocyphellaria. Two chemically noteworthy Australasian species are Pseudocyphellaria glaucescens, (found in eastern New South Wales and on Lord Howe Island) and Pseudocyphellaria rubella (found in Tasmania, southern Victoria and New Zealand). Each produces over 20 secondary metabolites. In the genus Pseudocyphellaria many species produce ten or more secondary metabolites, with some overlap, but not uniformity, in the chemistries of the different the species![]() .
.
Major classes of secondary metabolites
Secondary metabolites are synthesized via various chemical transformations starting from a few basic compounds. The transformations fall into three broad series or chemical pathways, with the pathways named after the relevant basic compounds. The table below lists the major classes of secondary metabolites within each pathway. This website will not go into any more detail about the biochemistry of lichens nor structural details of the secondary metabolites. For those with some biochemical knowledge, the table will give some idea of the biochemical processes within lichens. For those with no biochemical knowledge the terms in the following table will provide key words to use for finding further information.
 |
Direct evidence of biosynthesis within lichens (for example, via radioactively labelled isotopes) is still fairly meagre. However, a combination of the available direct evidence with indirect evidence (for example, laboratory experimentation and studies of biosynthesis in non-lichenized fungi) gives good support to the following table. Alongside each class of compounds is a bracketed number which is the approximate number of secondary metabolites of known structure in that class. As you can see if you do the arithmetic the number of secondary metabolites for which the chemical structures are known is significantly less than 700, meaning that there are many secondary metabolites for which the chemical structures have not yet been determined.
Acetyl-polymalonyl pathway
This pathway utilises acetyl-CoA and malonyl-CoA which are derivatives of coenzyme A.
Secondary aliphatic acids, esters and related derivatives (45)
Mononuclear phenolic compounds (19)
Depsides, tridepsides and benzyl esters (185)
Depsidones and diphenyl esters (112)
Depsones (6)
Dibenzofurans, usnic acids and derivatives (23)
Anthraquinones and biogenetically related xanthones (56)
Chromones (13)
Naphthaquinones (4)
Xanthones (44)Mevalonic acid pathway
Di-, sester- and triterpenes (70)
Steroids (41)Shikimic acid pathway
Terphenylquinones (2)
Pulvinic acid derivatives (12)
Roles of the secondary metabolites
While many secondary metabolites have known functions there are still many for which no function is known. This section will give some examples of the functions (or hypothesized functions) of some secondary metabolites.
The year 1944 saw the first published report of the discovery of antibiotic compounds in lichens. In that paper the authors found that 27 of the 42 lichen species they studied produced compounds that were effective against Staphylococcus aureus or Bacillus subtilis, four produced compounds that inhibited Proteus vulgaris or Alcaligenes fecalis but none of the tested lichen extracts inhibited Escherichia coli. Since that time many secondary metabolites have been shown to have antibiotic properties. This has been demonstrated in laboratory tests, with the metabolites generally most effective against fungi and gram positive bacteria. It is very likely that such compounds help protect lichens against a variety of micro-organisms in the wild, and so contribute significantly to the long life of many lichens, but there has been very little study to see what effects such compounds have in a natural setting. Laboratory studies have shown that several lichen metabolites inhibit the germination of, or the post-germination growth from, spores of various moss species. However various factors influence the toxicity of particular lichen metabolites. For example in laboratory studies that used the moss Funaria hygrometrica as a test subject, the toxicity of some lichen metabolites increased with increasing acidity but in others toxicity decreased with increasing acidity. Lichens and mosses grow on a variety of substrates, some of which will be more acidic and some more alkaline. Thus the nature of the substrate would influence the moss-inhibiting effectiveness of various lichen metabolites. Metabolites produced by some lichens may inhibit the growth of other lichens. An examination of a site in then Czechoslovakia found a species of the genus Lepraria growing on thalli of Xanthoparmelia loxodes and Xanthoparmelia verruculifera. Though the Lepraria was found on both Xanthoparmelia species, statistical analysis (which took into account the different numbers and sizes of the Xanthoparmelia thalli) showed a distinctly non-random distribution, with Lepraria overwhelmingly associated with Xanthoparmelia verruculifera. The host species were quite different chemically and the most plausible explanation for the non-random Lepraria distribution appeared to be chemical inhibition by Xanthoparmelia loxodes, even though the authors of the report about this phenomenon didn't carry out any laboratory experiments to test the chemical inhibition hypothesis. Chemical inhibition of mosses and other lichens would be advantageous given that they are often direct competitors for substrate. The secondary metabolites may also protect lichens against herbivorous invertebrates. Laboratory tests have shown that a number of invertebrates will avoid various lichen metabolites and that some lichen metabolites will induce slower growth rates in the invertebrates that have consumed them. Such compounds give clear survival benefits to the lichens involved![]() .
.
Some compounds in the upper cortices of lichen thalli reduce the intensity of the light reaching the photobiont cells. For example, photobionts in the genus Trebouxia (one of the commonest photobiont genera) function best in relatively low light. Lichens with such light filtering compounds can show variations in the concentrations of the compounds, with thalli growing in exposed locations having higher concentrations of filtering compounds than thalli growing in shaded locations. Carotenoids are pigments which vary from yellow to red in colour and which are found in a variety of organisms including a number of lichen genera such as Caloplaca ![]() and Xanthoria
and Xanthoria ![]() . Carotenoids play a number of roles. They help with photosynthesis (by absorbing light of wavelengths different to those absorbed by chlorophyll and transferring the captured light energy to the photosynthesis process) but also protect photosynthetic (and other) tissues against photo-oxidation by UV light. Carotenoid concentrations vary between species but even for a given species carotenoid production can vary depending on the amount of light where a thallus is growing. For example, a study involving the genus Xanthoria showed carotenoid content of 25 micrograms per gram of dry weight of thallus for Xanthoria fallax growing in a shady site and 71 micrograms for the same species in a sunny site. For Xanthoria parietina the corresponding figures were 48 and 63 micrograms. Experimental studies have shown that synthesis of parietin, a protective pigment in the lichen Xanthoria parietina, depended on UV-B radiation, with UV-A inducing little synthesis and photosynthetically active radiation inducing none
. Carotenoids play a number of roles. They help with photosynthesis (by absorbing light of wavelengths different to those absorbed by chlorophyll and transferring the captured light energy to the photosynthesis process) but also protect photosynthetic (and other) tissues against photo-oxidation by UV light. Carotenoid concentrations vary between species but even for a given species carotenoid production can vary depending on the amount of light where a thallus is growing. For example, a study involving the genus Xanthoria showed carotenoid content of 25 micrograms per gram of dry weight of thallus for Xanthoria fallax growing in a shady site and 71 micrograms for the same species in a sunny site. For Xanthoria parietina the corresponding figures were 48 and 63 micrograms. Experimental studies have shown that synthesis of parietin, a protective pigment in the lichen Xanthoria parietina, depended on UV-B radiation, with UV-A inducing little synthesis and photosynthetically active radiation inducing none![]() .
.
Identification via secondary metabolites
|
The secondary metabolites can be extracted from both fresh lichens as well as dried specimens that have been stored in herbaria for well over a century. For many lichens, but by no means all, chemical tests are essential for a correct identification. In routine identification work the simplest and quickest way to carry out such a step is by spot tests. In a spot test a tiny drop of a chemical reagent is applied directly to the lichen to see if there is any colour change. The standard chemical reagents for spot tests are solutions of iodine, potassium hydroxide, calcium hypochlorite and p-phenylenediamine. In the technical literature these are symbolised by I, K, C and P respectively so that if, for example, a particular lichen species is noted as K + yellow it means that the application of potassium hydroxide yields a yellow colour. The annotation K + yellow then red means that the application of potassium hydroxide first yields a yellow colour but after a little time there is a change to red. The annotation K - means there is no colour change with potassium hydroxide. The annotation KC + red means that first a drop of potassium hydroxide is applied and then a drop of calcium hypochlorite after which a red reaction is visible. Of course, just as KC means that first a drop of potassium hydroxide is applied and then a drop of calcium hypochlorite, the annotation CK indicates that the hypochlorite is applied first. A further point about spot tests is that it will at times be necessary to specify on what part of the lichen the test was done since certain compounds may be localized within the lichen. For example, to see a specimen annotated as medulla K + yellow means that part of the cortex and the photobiont layer have been carefully scraped away to expose the medulla to which a drop of potassium hydroxide has been applied, resulting in a yellow reaction.
Spot tests are very useful tools but they do not discriminate within broad classes of compounds. For example, potassium hydroxide turns bright red to purple with anthraquinone pigments but a red to purple result with a potassium hydroxide spot test gives no information as which anthraquinone pigment is present. Another important thing to note is that for a visual colour change the triggering secondary metabolite (or metabolites) need to be present in suitably high concentrations. If the metabolites are present in very low concentrations the metabolite molecules would still react in the standard way with the relevant spot test reagents but, given the small number of molecules involved, any colour change would be very hard, or even impossible, to detect with the naked eye.
Many secondary metabolites fluoresce under long wavelength ultraviolet light. However, as with spot tests, fluorescence does not discriminate within broad classes of compounds.
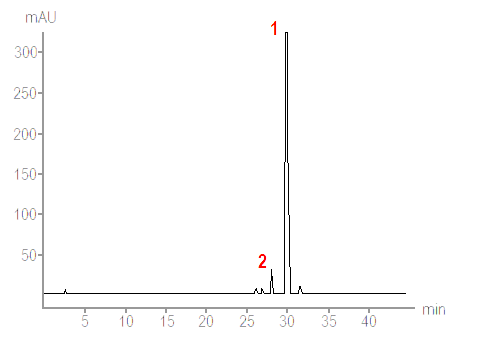
For more detailed analysis thin-layer chromatography (TLC) or high performance liquid chromatography (HPLC) are used. Depending on the type of chromatography an acetone extract from a lichen fragment is made to move in a solvent along a thin layer of silica gel on a firm supporting plate or through columns packed with tiny, spherical silica particles. Different lichen substances in the acetone extract move at different rates in the solvent and so become separated from each other, thereby allowing analysis and recognition of the different substances in a given lichen fragment - including substances that are present in low concentrations. That is a very simplistic description of chromatographic technique and anyone wanting more details will find them easily on the web via any search engine. All that matters here are, first, the basic principle and, second, that chromatographic methods (in particular HPLC) allow detailed analysis of the substances - not only what substances are present but also their relative proportions so seeing which are the major and which are the minor secondary metabolites in a particular lichen. The output of a HPLC system is typically a chart such as the following.
|
A technique such as HPLC is useful when analysing a puzzling lichen, especially if it is potentially a new species. In such a case as much and as precise information as possible is necessary. However chromatography has not rendered the simple spot tests obsolete. Though they lack the fine discrimination of chromatography, the battery of spot tests constitutes a simple and effective method for help in identifying many specimens that belong to already known species. Even in the case of a specimen that later turns out to be a new species some initial spot tests will still give a lichenologist some clues as to the affinities of the specimen in question.
Spot tests go back to the mid-19th century and for more about the early history go to the CHEMISTRY IN THE 1860s page.
Chromatographic analysis of old specimens
Chromatographic methods can be used to detect secondary metabolites in old herbarium specimens, sometimes very old specimens indeed. During the unwrapping of an Egyptian mummy in 1859 samples of Pseudevernia furfuracea that were found within the mummy were saved as herbarium specimens. Recently some of that lichen was analysed and, after 2,500 years, secondary metabolites were still detectable with HPLC, though results from thin layer chromatography had been negative. The HPLC analysis revealed physodic acid, isophysodic acid, atranorin, chloroatranorin and degradation products from physodic acid and/or oxyphysodic acid![]() .
.
Chemistry, species and genera
Nowadays no description of a lichen species would be considered complete without mention of the species' secondary metabolites - or a comment that the species is one of those lacking such metabolites. Chemical data is widely used in lichen taxonomy and chemical data is commonly used, along with other information, to help define generic limits. For example the genus Cetrelia lacks caperatic acid but all the species of the allied genus Platismatia produce that acid. This chemical difference is correlated with other morphological and distributional differences and, taken together, all the evidence justifies keeping the two genera distinct. The use of chemistry in differentiating species is where most controversy has arisen and there's more on the CHEMISTRY AND TAXONOMY page.
Lichen chemistry pages on this website Chemistry and taxonomy |
![An Australian Government Initiative [logo]](/images/austgovt_brown_90px.gif)