 Ecology
Ecology
Vagrant lichens
The great majority of lichens grow attached to substrates of some sort but many are able to continue growing if they become unattached. There is also a small number of species which never grow attached to any substrate. These are often referred to as vagrant lichens and I will use the word in that sense on this website. Species that normally grow attached but that are able to survive unattached are often referred to as erratic lichens, but be aware that some authors use the word erratic to include the vagrant species as well and then describe those species as obligatorily erratic. English words such as vagrant and vague are derived from Latin words built on the root VAG, conveying the sense of 'wandering'. In several other languages these lichens are known by names that are easy for an English speaker to understand. Thus they may be lichens vagabondes (French), licheni vaganti (Italian), líquenes migratorios, líquenes vagantes (both Spanish) or Wanderflechten (German). The last is easy to comprehend once you know that Flechten is the German word for lichens.
 Vagrant lichens are found in a variety of generally open, hot to cold, windswept habitats in widespread parts of the world - mallee or saltbush country in Australia, the Eurasian steppes, Arctic tundra, North American short-grass prairie, the low-altitude Namib Desert, high-altitude Andean páramo - and that list is not exhaustive. There are fewer than 100 vagrant species and they belong to several genera, especially Aspicilia and Xanthoparmelia. Here
Vagrant lichens are found in a variety of generally open, hot to cold, windswept habitats in widespread parts of the world - mallee or saltbush country in Australia, the Eurasian steppes, Arctic tundra, North American short-grass prairie, the low-altitude Namib Desert, high-altitude Andean páramo - and that list is not exhaustive. There are fewer than 100 vagrant species and they belong to several genera, especially Aspicilia and Xanthoparmelia. Here ![]() is Aspicilia fruticulosa, a vagrant fruticose lichen in a genus where the species generally have a crustose growth form. Aspicilia fruticulosa has been found in many areas of the Northern Hemisphere and vagrant Xanthoparmelias have been found in both hemispheres. At least seven vagrant species of Xanthoparmelia found in Australia. Most are endemic (such as Xanthoparmelia convoluta
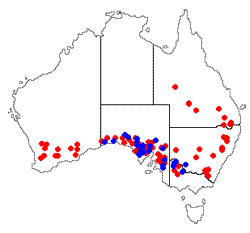
is Aspicilia fruticulosa, a vagrant fruticose lichen in a genus where the species generally have a crustose growth form. Aspicilia fruticulosa has been found in many areas of the Northern Hemisphere and vagrant Xanthoparmelias have been found in both hemispheres. At least seven vagrant species of Xanthoparmelia found in Australia. Most are endemic (such as Xanthoparmelia convoluta ![]() ) and found mostly in Australia's southern half. The accompanying map shows the distribution of Xanthoparmelia convoluta (blue dots) and Xanthoparmelia semiviridis (red dots). The latter is the most widespread vagrant species in Australia and is also found on New Zealand's South Island
) and found mostly in Australia's southern half. The accompanying map shows the distribution of Xanthoparmelia convoluta (blue dots) and Xanthoparmelia semiviridis (red dots). The latter is the most widespread vagrant species in Australia and is also found on New Zealand's South Island![]() .
.
Rolling & unrolling in foliose vagrants
Xanthoparmelia semiviridis and Xanthomaculina convoluta, both found in dry and often sandy areas, have foliose thalli that roll up as they dry but unroll and flatten when rehydrated. Here ![]() is the former when hydrated and here
is the former when hydrated and here ![]() it is dry. When flat the green upper cortex shows well. When dry the thallus rolls up to become somewhat ball-like and the upper cortex ends up on the inside of that 'ball'. The lobes of Xanthomaculina convoluta thalli roll up along their lengths so that when dry the lobes look like short black tubes, the black being the lower cortex
it is dry. When flat the green upper cortex shows well. When dry the thallus rolls up to become somewhat ball-like and the upper cortex ends up on the inside of that 'ball'. The lobes of Xanthomaculina convoluta thalli roll up along their lengths so that when dry the lobes look like short black tubes, the black being the lower cortex ![]() . Within a rolled up thallus that reveals only the lower cortex the photosynthesizing cells below the upper cortex would be protected from abrasion damage, such as that by wind-blown sand.
. Within a rolled up thallus that reveals only the lower cortex the photosynthesizing cells below the upper cortex would be protected from abrasion damage, such as that by wind-blown sand.
The medulla of Xanthoparmelia semiviridis, a species widespread in Australia's southern half, has loosely packed hyphae and so can accommodate the increasing volume of rehydrating hyphae without changing shape to any great degree. By contrast the hyphae of the upper cortex are densely packed, with very little or nothing in the way of gaps between the hyphae, even in the dry state. Consequently, when the thallus is rehydrated there is no space in the upper cortex to accommodate the expanding hyphae so the upper cortex expands more than does the medulla and the thallus unrolls![]() .
.
The thallus of Xanthomaculina convoluta, a species endemic to the coastal fog zone of the Namib Desert, has a structure with some resemblance to that of Xanthoparmelia semiviridis. The hyphae of the cortex are densely packed and the medullary hyphae are loosely packed. However there are intrusions of the medulla and the algal layer into the upper parts of the thallus so that (unlike the case in Xanthoparmelia semiviridis) the cortex is not continuous. The following pair of stylized diagrams show cross sections of a thallus when dry (left) and when wet (right). The upper cortex has two layers. The upper layer, called the epicortex, is thin and shown in blue. Below is the bulk of the cortex (in dark grey), the algal cells (green), the medulla (speckled light grey) and the lower cortex (black).
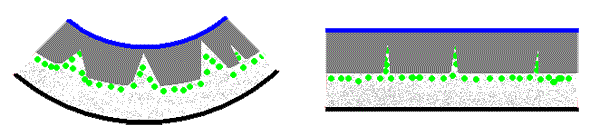
The intrusions show clearly in the dry thallus and you can see that some reach the epicortex and some don't. When wet these are compressed and hence not as noticeable.You'll find photographs of cross sections of dry and wet thalli here. As in Xanthoparmelia semiviridis there is differential swelling, with the gaps in the medulla of Xanthomaculina convoluta also able to accommodate the rehydrated medullary hyphae, whereas there is no expansion room in the cortex. Hence the thallus unfolds with the intrusions acting somewhat as hinges for the dark grey cortex areas. The presence of calcium oxalate crystals on the medullary hyphae gives some rigidity to the Xanthomaculina medulla. This results in the unfolded Xanthomaculina thallus being flat whereas in Xanthoparmelia semiviridis the expanding cortex can result in the thallus of that lichen unfolding so far as to be curved slightly backwards![]() .
.
On this page of the University of Trieste website there is a brief account of Xanthomaculina convoluta . The text is in Italian but if you cannot read Italian you can at least enjoy the photographs showing thalli of this species in situ and read a rough translation in the following reference button![]() .
.
Reproduction
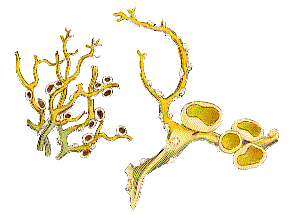
Vagrant lichens appear to produce apothecia rarely. In a paper of 1993 the author noted that amongst the seven vagrant species of Xanthoparmelia found in North America only two (Xanthoparmelia chlorochroa and Xanthoparmelia camtschadalis) had been reported with apothecia and then very rarely. Reproduction was by unspecialized fragmentation. The photo (right) shows apothecia of Xanthoparmelia semiviridis but they are rarely found, though thallus fragments can be found often. Amongst the other vagrant Xanthoparmelias found in Australia rare apothecia have been found only on Xanthoparmelia convoluta and Xanthoparmelia sorediata. In the early years of the 20th century the Russian lichenologist Konstantin Mereschkowski collected vagrant lichens in the Crimea. He stated that he'd collected several thousand specimens of vagrant Parmelia and had seen even more but had never come across a single specimen with an apothecium and nor had he ever seen soredia. Reproduction was by a thallus breaking into two or three pieces. With regard to Aspicilia fruticulosa Mereschkowski reported finding apothecia on only two or three specimens of the over ten thousand he'd seen. No apothecia have been reported for Xanthomaculina convoluta which may reproduce by irregular fragmentation or by the phyllidia found on the thalli. A phyllidium is a small, leaf-like projection from the thallus, with distinct upper and lower surfaces and often constricted at the base, easily broken off and discarded to function as a vegetative propagule. Masonhalea richardsonii (a vagrant species of Siberia, Alaska and northern Canada) has rarely been found bearing apothecia![]() .
.
Thalli of vagrant lichens may be broken by being stepped on or by being forcibly blown against obstacles by the wind. Mereschkowski also wondered about the effect of repeated wetting and drying and reported the following:
I carried out the following experiment to see if thallus fragmentation was helped by rain, the consequential swelling and the later shrinkage upon drying. I chose a hundred specimens of Aspicilia affinis that appeared ready for division. I watered them each morning with an atomiser and then let them desiccate. About a month later a number of specimens had divided, with an increase of about 20 to 30 to the number of specimens. I cannot give the exact number since I don't have my notes, which are still in Russia
.
When it comes to the dispersal of fragments or phyllidia the answer may be ...
...blowing in the wind
It had long been suggested that vagrant lichens were dispersed by wind, perhaps as whole thalli or as fragments. In this section I summarize some of the findings from two studies into the possibility of wind dispersal of vagrant lichens.
In one study specimens of Xanthoparmelia vagans were collected at an altitude of 4530 metres in the Sierra de La Culata, Venezuela. Several thousand thalli occupied an area of about 100 square metres that was almost devoid of vascular plants. All the thalli identifiable as Xanthoparmelia vagans were collected from a 2 square metre area and this yielded 166 specimens. These were taken back to the laboratory for various tests and experiments. The following reference button gives some summary information about the sizes of those 166 specimens![]() .
.
Thirty-one dry specimens, with dry weight from 0.04 to 3.63 grams, were used in an experiment to test wind transport of thalli placed on three types of surface: a flat board and two types of corrugated styrofoam to simulate the rough surfaces of the area where the lichens had been collected. One of the styrofoam surfaces had an 8 to 10 millimetre relief (ie, the distance between the surface's low and high points) and the other a 40 millimetre relief. The wind speed needed to get specimens moving (called the threshold speed) depended on lichen mass and surface roughness. A few of the specimens had an elongated shape and it was found that when such specimens were placed with their narrow side facing the wind, the threshold speed was about half a kilometre per hour greater than when the specimens were placed with their broad side facing the wind. For the series of experiments all elongated specimens were placed with their broad sides facing the wind.
For the mathematically inclined, if y denotes the threshold speed in kilometres per hour and x the weight of a lichen in grams, then the following quadratic equation is a good predictor of threshold speed on a smooth surface, for the weight range given above: |
Over the flat surface small lichens could be mobilized by winds of about 2.5 kilometres per hour while the largest specimens needed wind speeds of over 17 kilometres per hour. Generally, once moving, the specimens would continue to do so. On the styrofoam with the shallower relief the threshold speeds were about 40% higher. Moreover, in the experiment some of the larger specimens either were not moved or were easily stopped by the roughness. However, the equipment available could not generate wind speeds greater than about 18 kilometres per hour and it is likely that higher wind speeds would have moved the larger specimens. On the rougher styrofoam several specimens were moved but all were quickly caught by the surface depressions so no effective wind transport took place. Wind speeds of 30 to over 40 kilometres per hour had been recorded at the collection site, but the higher speeds were more common during the rainy season. At that time the lichens would be saturated and considerably heavier so there would be no guarantee that effective wind transport could take place at that time. The author of the study suggested that effective wind transport took place during occasional episodes of strong winds during the dry season.
An Australian study, using a portable field wind tunnel, looked at the effect of wind on thalli of Xanthoparmelia semiviridis, also placed on three different surfaces: (1) a bare board (2) a soil surface with a BIOLOGICAL SOIL CRUST composed of crustose and squamulose lichens and mosses, covering about 32% of the surface and (3) a crusted surface covered with a layer (about three millimetres deep) of Casuarina cristata branchlets. The branchlets are no more than about a millimetre in diameter and can be many centimetres long, thus superficially needle-like, but soft. The experiments took place at Tapio Station, near Buronga in south-west New South Wales. The experimenters used 50 dry thalli and, though the thallus masses were not given, the size information given in the published report implies that the majority of thalli would have had diameters between 15 and 19 millimetres. There is a mathematical formula to allow the conversion of a near-surface wind speed (which was measured in the wind tunnel) to what the wind speed would be at a greater height above the surface. Much information about wind speeds 10 metres above the surface is available from Australia's Bureau of Meteorology and, in order to allow comparison of the wind tunnel data with information from the Bureau, the experimenters converted the wind tunnel speeds to equivalent speeds at a height of 10 metres above the ground. It is the converted speeds that were published and that are given below.
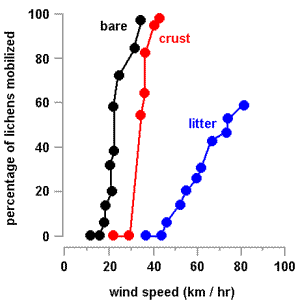 The wind speeds needed to start movement were about 17.5 kilometres per hour (on the bare surface), 34.6 kilometres per hour (crust surface) and 46.0 kilometres per hour (on the Casuarina litter). On both the bare and crusted surfaces once some thalli had started moving it took only small increases in wind speed to move all the thalli. By contrast, on the Casuarina litter, the highest attainable wind speed of 82 kilometres per hour was unable to move about 40% of the thalli. The graph (right) shows the percentages of thalli moved at various wind speeds. A word of warning is necessary for the 'litter' graph. In the experiment the thalli were placed on the litter surface. Normally when hydrated thalli dry and curl up amongst leaf litter the thallus lobes would often wrap around or become entangled with the litter, thereby making thalli harder to move. Consequently it is likely that often wind speeds higher than indicated in the graph would be needed to move thalli.
The wind speeds needed to start movement were about 17.5 kilometres per hour (on the bare surface), 34.6 kilometres per hour (crust surface) and 46.0 kilometres per hour (on the Casuarina litter). On both the bare and crusted surfaces once some thalli had started moving it took only small increases in wind speed to move all the thalli. By contrast, on the Casuarina litter, the highest attainable wind speed of 82 kilometres per hour was unable to move about 40% of the thalli. The graph (right) shows the percentages of thalli moved at various wind speeds. A word of warning is necessary for the 'litter' graph. In the experiment the thalli were placed on the litter surface. Normally when hydrated thalli dry and curl up amongst leaf litter the thallus lobes would often wrap around or become entangled with the litter, thereby making thalli harder to move. Consequently it is likely that often wind speeds higher than indicated in the graph would be needed to move thalli.
Data from the Bureau of Meteorology indicated that during summer the Tapio Station area was likely to experience wind speeds above the bare board threshold about 21.5% of the time. A bare board may seem an artificial surface but it would equate to the surfaces of the many bare soil scalds in the study area. The Bureau data indicated also that wind speeds above the crust threshold would occur about 1.25% of the time in summer and speeds above the Casuarina litter threshold during less than 0.5% of summer. Thus, away from the scalds, the Bureau's data indicates that, for any given summer, there are likely to be only a few days when Xanthoparmelia semiviridis thalli could be moved by the wind in a soil crust area and, where there is Casuarina litter, the right conditions for thallus movement are likely to occur for no more than a few hours each summer. It seems likely that a given thallus on Casuarina litter would move only once every few years.
The conclusions of the previous paragraph relate to intact thalli. The wind tunnel experiments also showed that continual bombardment of Xanthoparmelia semiviridis thalli against the soil surface or against any obstructions above the soil surface tended to break the thalli into smaller fragments, which were much easier to move. Moreover, many such fragments were not easily trapped by litter and so could function as longer distance propagules. With regard to breakage the author of the South American study reported that the thalli of Xanthoparmelia vagans were mostly firm and could withstand a fair amount of handling, but some of the larger specimens were breaking up into two or more pieces at the collection site. The tendency was for elongated thalli to break up into more globular thalli![]() .
.
|
Though wind may be capable of moving many thalli the experiments reported above leave unanswered the question of the effectiveness of wind dispersal. In many cases wind-blown thalli are likely to be stopped by obstructions such as vegetation, piles of leaf litter, fallen trunks or man-made obstacles such as drainage ditches alongside roads. Thalli of vagrant lichens can be found piled up at such obstructions and further dispersal may well be difficult to impossible (for example, from the bottom of a roadside ditch. Moreover, in any such accumulation the lowermost thalli may be covered so well by the upper thalli and leaf litter as to die. In such conditions the lowermost thalli would be deprived of the light necessary for photosynthesis and the moisture content may be enough to lead to rotting of the thallus.
Other possible means of dispersal
It is possible that thallus fragments or small thalli could be caught in animal fur and so be transported over much longer distances than is possible by wind. In North America sheep have been found to eat vagrant lichens. Pronghorn antelopes have also been recorded as using vagrant lichens and it has been pointed out that the distribution of vagrant species of Xanthoparmelia is very similar to the pronghorn antelope distribution. Given such activities it is not hard to imagine thalli or fragments being picked up by these (or other) animals as they rummage or rest in areas where vagrant lichens occur![]() .
.
Water has also been suggested as a means of dispersal and certainly dispersal by water is likely to occur in some circumstances. However the authors of the Tapio Station study warned against assuming that dispersal by water was common in the gently sloping landscapes found in many parts of semi-arid Australia, though short busts of intense rain do occur in many such areas. They looked at litter that had been piled up by the surface flows that follow intense rain to see if such piles contained Xanthoparmelia semiviridis but were unsuccessful. It is possible that smaller thalli or fragments could be missed. There is also the question as to how quickly thalli that became embedded in such piles could survive, without sunlight, before decaying and so not being recognizable. However, if water transport were common in the areas surveyed by the authors it would be reasonable to expect thalli to be deposited also on or near the surfaces of such piles so the authors may well be right when they suggest that thalli of Xanthoparmelia semiviridis are not easily moved by water at all locations.
In various cold areas ice crystals form overnight in moist soil, leading to expansion of the ice-impregnated soil. During the daytime the crystals melt but the next night a new lot of ice crystals forms (such as the needle ice mentioned in the next section). Over time this phenomenon of frost heave can lead to a very noticeable upward thrust of the soil surface and any raised ball of vagrant lichen may become unbalanced enough to roll, but movement induced by frost heave is likely to lead to very localised dispersal.
Xanthoparmelia vagans - water gain, water loss and needle ice
The specimens of Xanthoparmelia vagans collected at the Venezuelan location were tested also for water gain and loss. Water absorption occurred fairly quickly. When dry thalli were placed in 2 to 3 millimetres of water it took only a few seconds for the rising water front to reach the top of even the largest specimen and full turgidity occurred within 2 to 3 minutes. After becoming saturated thalli were allowed to dry until again desiccated (defined as having a water content less than 1% of the dry thallus weight). Fully saturated thalli held from one and a half to a little over three and a half times their own dry weight in water. As a general rule the smaller lichens held the larger percentages but there was not a strong relationship between percentage and dry weight. When saturated thalli were allowed to dry the rate of water loss was determined by dry weight and not by water content of the saturated thallus. The ten smallest specimens by dry weight become desiccated within 8.5 hours, whereas the ten largest needed between 17 and 42.5 hours.
In the Venezuelan collection site needle ice is common at many times of the year. As long as there is sufficient moisture in the soil, capillary action during night-time freezing draws water from lower soil levels towards the surface where ice forms in the shape of needles, possibly densely packed. In the morning the melting ice yields a surface water layer that would be readily absorbed by lichens. It is possible that water from needle ice is an essential source of moisture for Xanthoparmelia vagans.
Lichens need to be moist (but not too wet) to maximize the rate of photosynthesis. Studies have shown that moisture contents of 65% to 90% of total saturation are required for maximum photosynthesis rates in various species. If this holds for Xanthoparmelia vagans the smaller specimens need not benefit from a high water-holding ability. It is possible that persistence of moisture is more important. When a dry lichen rehydrates it begins to metabolize almost immediately but respiration rises more quickly than photosynthesis. Until photosynthesis has reached a level able to compensate for respiration the lichen is using its metabolic reserves that have been produced during previous periods of photosynthesis. Hence a series of quick dry-wet-dry episodes could be detrimental or allow for only slow growth, since reserves could be used at a faster rate than that at which they could be replenished. The small thalli of Xanthoparmelia vagans could moisten each morning (for example, from melting ice crystals) photosynthesize for some time but then start drying as the sun rose higher and be totally dry before the day's end. By contrast the larger thalli still held from 20 to 50 percent of dry weight in water, 24 hours after saturation, and if the laboratory results held in the wild then such thalli would not start each day in a dry state. Hence, they could more readily make use of each morning's water and maintain a net positive photosynthetic output indefinitely.
![An Australian Government Initiative [logo]](/images/austgovt_brown_90px.gif)



